Hemlibra® (emicizumab-kxwh) is an antibody indicated to reduce the frequency of bleeding episodes in adults and children with haemophilia A with factor VIII inhibitors.
Developed by Genentech, Hemlibra’s biologics license application (BLA) was accepted for review by the US Food and Drug Administration (FDA), which granted the drug priority review designation in August 2017.
The FDA approved the drug in November 2017 for routine prophylaxis for the prevention or reduction of the frequency of bleeding episodes in adults and children with haemophilia A with factor VIII inhibitors.
Hemlibra is currently under review by the European Medicines Agency (EMA) for marketing authorisation in Europe. The EU Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion for the approval of Hemlibra® in January 2018.
Other medications available in the market for the same indication include Bayer’s Kovaltry and Octapharma USA’s Nuwiq.
Haemophilia A causes
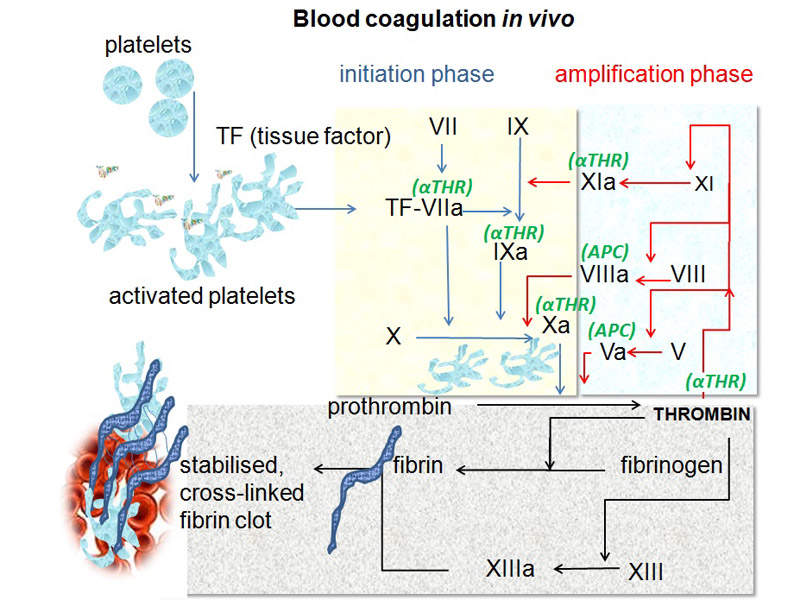
Haemophilia A is a genetic disorder that causes insufficient blood clotting, leading to uncontrolled and often spontaneous bleeding due to lack of clotting protein factor VIII. The disease affects joints and muscles, as well as often causing pain. It can lead to chronic swelling, deformity, reduced mobility and long-term joint damage.
The disease affects approximately 320,000 people worldwide, and around 60% suffer from a severe form of the disorder.
Hemlibra’s mechanism of action
Hemlibra® contains bispecific factor IXa and factor X-directed antibody. These restore the function of missing activated factor VIII, which is necessary for effective haemostasis.
The drug is available in 150mg/ml dose for subcutaneous use.
Clinical trials on Hemlibra
Genentech conducted a Phase III clinical trial known as HAVEN 1 to evaluate the safety and pharmacokinetics of Hemlibra®. It was a randomised, multi-centre, and open-label study that enrolled 109 patients, including adults and adolescents with haemophilia A with inhibitors to factor VIII that were previously treated with bypassing agents (BPA) on-demand, or as prophylaxis.
The patients were randomised to receive either Hemlibra prophylaxis or no prophylaxis in a 2:1 ratio. The patients that received prior treatment with BPAs as prophylaxis received Hemlibra® prophylaxis, while additional patients treated before with on-demand BPAs were also enrolled in a separate arm.
The primary endpoint of the study was met, demonstrating that patients treated with Hemlibra® prophylaxis showed statistically significant reduction in treated bleeds of 87% when compared to no prophylaxis.
The study also showed that 62.9% of the additional patients that received Hemlibra® prophylaxis experienced zero treated bleeds compared with the 5.6% of patients that received no prophylaxis.
A second Phase III clinical trial known as HAVEN 2 was conducted on Hemlibra®. It was a single-arm, multi-centre, open-label clinical study that enrolled children younger than 12 years with haemophilia A with inhibitors to factor VIII. It evaluated the efficacy, safety and pharmacokinetics of Hemlibra prophylaxis.
The median observation time for the study was 38.1 weeks. Interim analysis demonstrated that 87% of the children that received Hemlibra prophylaxis experienced zero treated bleeds.
The study also showed that 34.8% of children experienced zero bleeds overall, which included all treated and non-treated bleeds. In addition, 95.7% of children experienced zero treated spontaneous bleeds and zero treated joint bleeds, while 100% of children experienced zero treated target joint bleeds.
The most common adverse events reported during the clinical study were injection site reactions, headaches, and joint pain. These events occurred in 10% or more of patients treated with Hemlibra in pooled studies.
A third Phase III clinical trial known as HAVEN 3 evaluated Hemlibra® prophylaxis dosed once weekly or once every other week in people aged 12 years or older with haemophilia A without inhibitors to factor VIII.
HAVEN 3 was a randomised, multicentre, open-label, Phase III study that enrolled 152 patients with hemophilia A. The patients were randomised to receive subcutaneous Hemlibra® prophylaxis either every week or every two weeks. The results showed a statistically significant reduction in treated bleeds compared to no prophylaxis.
Another Phase III clinical study named HAVEN 4 is evaluating Hemlibra® prophylaxis dosed every four weeks in people aged 12 years or older, with haemophilia A with or without inhibitors.