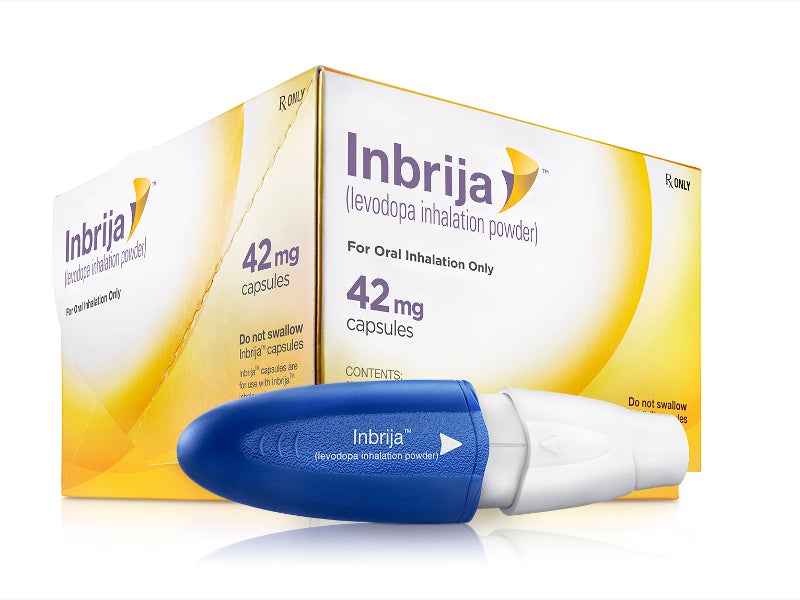
Inbrija® (levodopa inhalation powder) is an oral formulation indicated for the intermittent treatment of OFF episodes in Parkinson’s disease patients treated with a combination of carbidopa and levodopa.
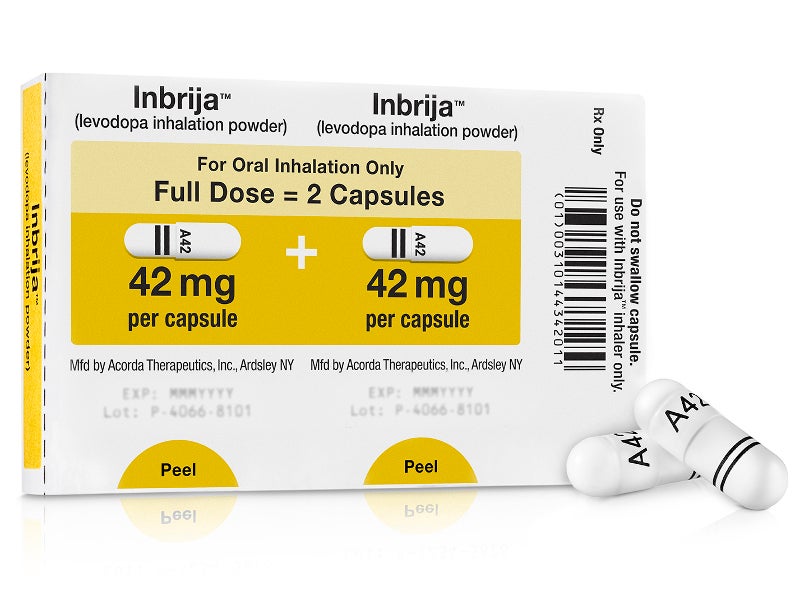
Developed by Acorda Therapeutics, Inbrija contains a dry powder formulation of levodopa for oral inhalation with the Inbrija inhaler. The inhalation powder is packed as white hypromellose capsules comprising 42mg of spray-dried levodopa powder together with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and sodium chloride.
Acorda collaborated with Spanish pharmaceutical company Esteve Pharmaceuticals for the distribution and supply of Inbrija 33mg (levodopa inhalation powder, hard capsules) in Spain and Germany in July 2021 and November 2021, respectively. Esteve launched Inbrija 33mg in Germany in June 2022, while the drug’s commercial launch in Spain is expected in early 2023.
In May 2022, Acorda entered distribution and supply agreements with Biopas Laboratories, a pharmaceutical company based in Colombia, to commercialise Inbrija in Latin America.
Regulatory approvals for Inbrija
The US Food and Drug Administration (FDA) raised issues with the drug as part of an initial new drug application (NDA) submitted by Acorda Therapeutics. The company addressed these points and resubmitted the application in December 2017.
The NDA was then accepted for review in February 2018 and the drug was approved in December 2018, making it one of the first FDA-approved drugs for its indication.
In March 2018, Acorda submitted a marketing authorisation application (MAA) for the drug to the European Medicines Agency (EMA). The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion recommending the drug’s approval in the European Union (EU) in July 2019.
The European Commission (EC) approved Inbrija in September 2019.
Inbrija typically works more quickly than pills that are orally ingested.
Parkinson’s disease causes and symptoms
Parkinson’s disease is a progressive neurodegenerative condition caused by the gradual loss of nerve cells in the brain. This leads to the decline of brain chemical dopamine levels and causes various symptoms.
More Parkinson’s cases occur in men than women and most patients are aged 55 to 65 years. The risk of the disease increases with age and its symptoms worsen with time.
Common symptoms of Parkinson’s disease include difficulty in body movements such as walking and talking, stiffness, tremor, and loss of balance and coordination. The gradual progression of the disease may cause disease symptoms to recur in patients who are on therapy; this is known as OFF episodes, the episodic motor fluctuations in patients.
An estimated one million individuals in the US and 1.2 million in Europe are diagnosed with the disease each year. OFF episodes are experienced by around 40% of the patients diagnosed with the disease in the US.
Inbrija’s mechanism of action
Inbrija uses Acorda’s innovative ARCUS® pulmonary delivery system, a technology platform designed to deliver medication through inhalation.
The brain cells of a Parkinson’s patient begin to die and their dopamine level decreases, causing difficulty in muscular movement. Levodopa, the metabolic precursor of dopamine, is absorbed through the lungs when inhaled, before crossing the blood-brain barrier and being converted to dopamine in the brain.
Inbrija typically works more quickly than pills that are orally ingested. It can be taken when required or during the return of symptoms, generally five times a day, alongside Parkinson’s medications.
Clinical trials on Inbrija
The FDA’s approval of Inbrija was based on the positive results of a clinical development programme, which included two Phase III clinical trials.
The first trial was a pivotal, randomised, placebo-controlled, double-blind 12-week Phase III clinical study named SPANSM-PD, which was conducted to evaluate the drug’s efficacy in Parkinson’s disease patients experiencing OFF episodes.
Of the 351 patients enrolled in the study, 114 received 84mg of Inbrija and 112 patients were given placebo. The trial’s primary endpoint changed in Unified Parkinson’s Disease Rating Scale (UPDRS) part III motor score, which signifies an improvement in the patient’s motor function.
At week 12, the mean change in score after 30 minutes of dosing with Inbrija was -9.8 points compared with -5.9 points in patients receiving placebo.
The second Phase III trial was a 12-month randomised, controlled, open-label study that evaluated Inbrija’s effects on pulmonary function. Of the total 408 participants that were enrolled in the clinical trial, 271 received 84mg of Inbrija and 127 were put in the control group and given their regular oral medication regimen.
Patients receiving Inbrija showed an average reduction in forced expiratory volume in 1s compared with those in the control group.
Common adverse events reported in patients during the clinical trials included coughing, nausea, upper respiratory tract infections, and discoloured sputum.