Under development by specialist pharmaceutical company, Neurogen, NG2-73 is a new, selective gamma-amino butyric acid (GABA) partial agonist indicated for the treatment of insomnia, the most common type of sleep disorder. The drug has now been renamed Adipiplon. Phase II development initial clinical results suggested that NG2-73 has the capacity to reduce sleep latency as well as increase total sleep time and sleep efficiency in insomnia patients.
NG2-73 targets the Gaba alpha-3 receptor subtype
Exclusive rights to the GABA programme are held by Neurogen. Drugs that act via GABA receptors represent a new approach to the treatment of sleep disorders. Neurogen’s NG2-73 acts as a partial agonist at the GABA alpha-3 receptor subtype, where it stimulates the receptor to enhance neurotransmitter activity.
Action at one of the five GABA receptor subtypes (alpha-1 to alpha-5) can elicit different pharmacological effects. Activity at the alpha-1 receptor subtype is associated with sedation but also memory impairment and possible abuse potential, whereas action at the alpha-2 receptor subtype produces anxiolytic and muscle relaxing effects. In contrast, the alpha-3 receptor subtype is associated with sleep inducing, hypnotic and anxiolytic effects, making it an appropriate drug target for sleep disorders.
Potential in transient and chronic insomnia
NG2-73 has shown early evidence for clinical efficacy in a Phase II study in transient insomnia. In this randomised, placebo-controlled trial of 369 patients with transient insomnia, treatment with NG2-73 produced a significant time reduction in the onset of persistent sleep compared with placebo (p<0.0001). At all doses tested, NG2-73 was well tolerated. There were no serious treatment-related adverse events or drug-related withdrawals from the trial.
Phase II trials are now underway to study the therapeutic effects of NG2-73 in chronic insomnia. The largest of these, a randomised, placebo-controlled 240-patient trial, will explore the effects of five different doses of NG2-73 on sleep onset as defined by latency to persistent sleep (primary endpoint) as well as sleep maintenance.
The top line results of Phase II b trials were announced in June 2007. They were followed by the combination of three controlled releases and an immediate release of adipiplon. These trials were conducted on chronic insomnia patients.
The Phase II/III trial of bilayered formulation of adipiplon versus Ambien CR was suspended because of high after-effects in chronic insomnia patients. The company has said that it did not have any immediate plans to extend its research activities in this area.
Expanding treatment options for sleep disorders
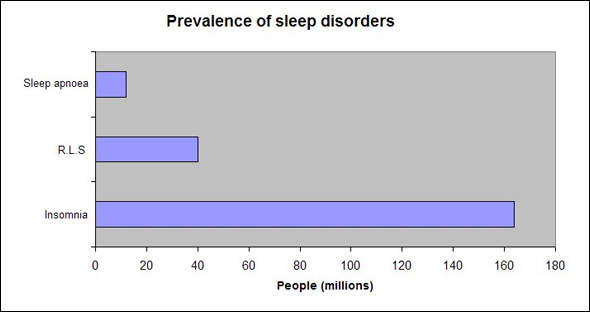
Estimated to affect between 20% and 30% of the general population, sleep disorders are extremely common. Treatments range from self-medication with non-pharmaceutical preparations to prescription medications, such as benzodiazepines, antidepressants, antihistamines and most recently, selective hypnotics.
Although the new generation of selective or dedicated hypnotic agents represent a major advance in the treatment of insomnia, they still carry some risk of dependence.
Ideally, drug therapy for insomnia should induce sleep that is qualitatively close to normal sleep cycles and be devoid of rebound and withdrawal effects.
GABA agonists represent a promising new class of drugs for the treatment of sleep disorders, hopefully delivering an improved side-effect profile over existing agents and minimal potential for abuse. This would be a major selling point if drugs in this class are approved and gain market entry.
Marketing commentary
For years, conventional benzodiazepines were the mainstay of pharmacological treatments of insomnia. They have been gradually replaced by the new selective hypnotics, which now dominate and drive the market for sleep disorder treatments. Although efficacious they can cause adverse effects such as ‘hangovers’, rebound insomnia and, more rarely, short-term memory loss.
Sleep disorders remain poorly understood, under-diagnosed and under-treated. Good opportunities exist for new drugs to treat sleep disorders, especially where they offer benefits over existing agents.