Invega Hafyera™ (paliperidone palmitate) is the first and only twice-yearly dosing agent indicated for the treatment of adults with schizophrenia.
The drug was developed by Janssen Pharmaceuticals, a Belgium-based pharmaceutical company owned by Johnson and Johnson. It contains a racemic mixture of (+) and (-) paliperidone palmitate.
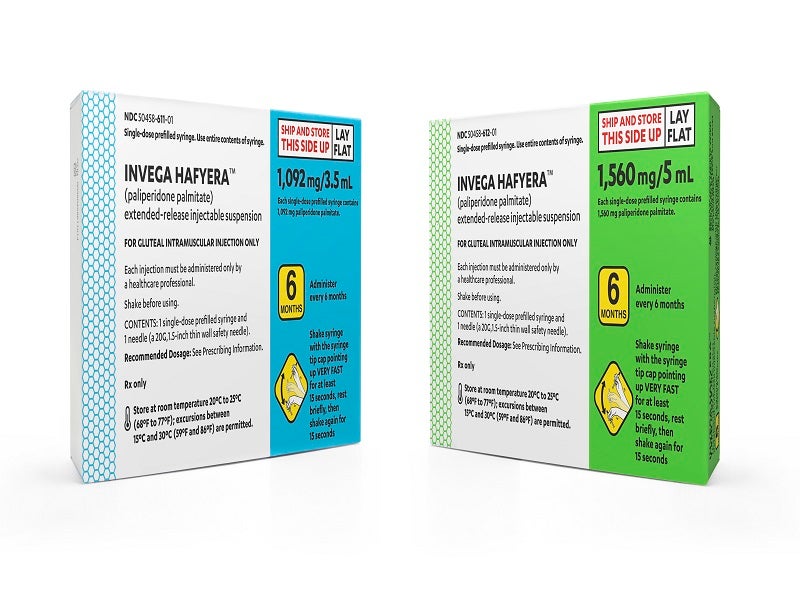
Invega Hafyera is available as a white to off-white aqueous extended-release injectable suspension in 1,092mg/3.5ml and 1,560mg/5ml dosage strengths in single-dose prefilled syringes for gluteal intramuscular injection.
Regulatory approvals for Invega Hafyera
In November 2020, Janssen submitted a supplemental new drug application to the US Food and Drug Administration (FDA) for paliperidone palmitate six-month (PP6M) for the treatment of adults diagnosed with schizophrenia. The drug was previously approved by the FDA for the acute and maintenance treatment of schizophrenia in 2006.
In December 2020, Janssen submitted a marketing authorisation extension application to the European Medicines Agency (EMA) to register PP6M injectable product for the maintenance treatment of schizophrenia in adult patients who are clinically stable and receiving paliperidone palmitate one-monthly (PP1M) or three-monthly (PP3M) formulations.
Invega Hafyera (six-monthly paliperidone palmitate) received FDA approval for the treatment of schizophrenia in adults in September 2021.
Schizophrenia causes and symptoms
Schizophrenia is a chronic and severe mental disorder that affects an estimated 20 million people worldwide and 2.8 million adults in the US. The condition may be caused by a combination of genetic and environmental factors. Psychosocial factors may also influence the occurrence of schizophrenia.
The disease is characterised by distortions in thinking, perception, emotions, language, sense of self, and behaviour.
Common experiences include hallucinations, such as hearing voices or seeing non-existent objects, delusions, and abnormal behaviour such as aimless walking or laughing at oneself, self-neglect or seeming untidy, as well as disorganised speech such as irrelevant speech, and emotional disturbances such as noticeable indifference. It can also cause neurological damage and severe disability.
Medications and psychological assistance are effective treatments for schizophrenia.
Invega Hafyera’s mechanism of action
The long-acting injectable drug is administered every six months in the upper buttocks area and slowly dissolves into the bloodstream, providing six months of continuous treatment and symptom control.
Paliperidone palmitate is hydrolysed to paliperidone, the major active metabolite of risperidone.
Paliperidone’s mechanism of action is unknown, but its effectiveness in the treatment of schizophrenia may be mediated through a combination of central dopamine D2 and serotonin 5HT2A receptor antagonism.
Clinical trials on Invega Hafyera
Invega Hafyera’s efficacy in treating schizophrenia was evaluated in a 12-month randomised, double-blind, active-controlled, interventional, parallel-group, multi-centre, non-inferiority Phase III global clinical trial.
The study enrolled 702 schizophrenic patients who were stably treated previously with either Invega Sustenna (PP1M) for at least four months or Invega Trinza (PP3M) for at least one three-month injection cycle, or injectable risperidone or any other oral antipsychotic. The trial’s primary endpoint was the time to relapse in individuals with a DSM-5 diagnosis of schizophrenia.
In the clinical study, the patients were randomised at a 2:1 ratio to receive either Invega Hafyera (478 patients) or Invega Trinza (224 patients).
Invega Hafyera was found to be non-inferior to Invega Trinza at the end of 12 months, with 92.5% of patients treated with Invega Hafyera and 95% of patients treated with Invega Trinza being relapse-free after 12 months.
The most frequent adverse reactions reported in patients during the clinical study were upper respiratory tract infection, injection site reaction, weight gain, headache, and parkinsonism.