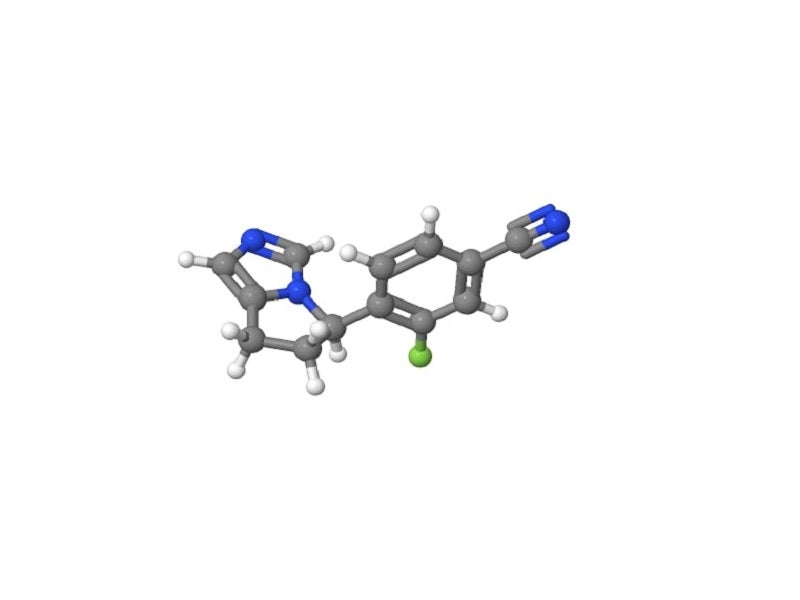
Isturisa® (osilodrostat) is the first cortisol synthesis inhibitor indicated for the treatment of Cushing’s disease in adult patients ineligible or failed pituitary surgery.
Recordati, a pharmaceutical company based in Italy, acquired the worldwide rights to the drug, developed by Novartis, in October 2019.
Isturisa received orphan drug designation from the US Food and Drug Administration (FDA) in September 2013 and the European Medicines Agency (EMA) in October 2014. Novartis submitted a new drug application (NDA) for the drug to the FDA in March 2019, approved in March 2020.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) recommended the drug for marketing in the European Union for Cushing’s syndrome, in November 2019. The drug received the marketing authorisation from the European Commission (EC) in January 2020.
Isturisa is available as oral, round, unscored, and biconvex tablets in three dosage strengths of 1mg, 3mg, and 5mg. The 1mg tablet is pale yellow coloured, while the 5mg tablet is yellow and the 10mg tablet is pale orange-brown.
Cushing disease causes and symptoms
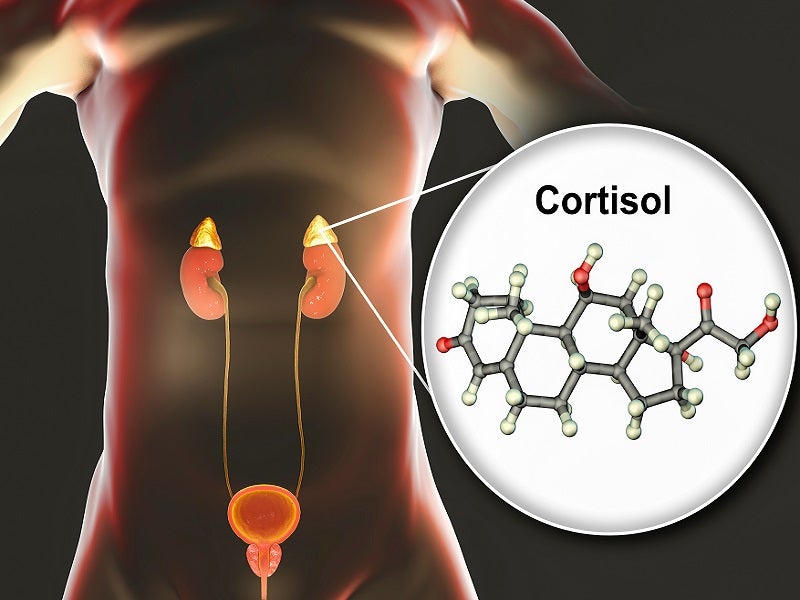
Cushing’s disease is a severe, rare endocrine disease. When the excessive adrenocorticotropic hormone is released due to enlargement of the pituitary gland or by a pituitary tumour, the adrenal glands get stimulated to produce a large amount of cortisol, which causes the Cushing’s disease.
The disease is most likely to occur between 30 and 50 years of age. It affects women three times more than men, caused by various health conditions including high blood pressure, type-2 diabetes, central obesity, blood clotting in legs and lungs, compromised immune system, bone loss and depression.
Characteristic features of patients include thin arms and legs, increased fat around the neck area, round red face, weak muscles, prone to bruising and purple stretch marks.
Mechanism of action of Isturisa
Isturisa is an inhibitor of 11-beta-hydroxylase enzyme crucial for the cortisol biosynthesis in the adrenal glands, preventing the synthesis of cortisol.
Clinical studies of Isturisa
Isturisa’s approval by the FDA comes from positive outcomes of a clinical programme LINC. Majority of the patients in the programme demonstrated normalisation of the cortisol levels and improvement in various clinical features of the Cushing’s disease.
A total of 137 patients enrolled in phase III clinical study, LINC-3 to evaluate the long-term efficacy and safety of osilodrostat in patients with Cushing’s disease. The study is a multi-centre, double-blind, randomised withdrawal study post the single-arm, open-label, dose titration and maintenance treatment for 24 weeks, which determined the safety and efficacy of the drug. The primary goal of the study was to control cortisol level in patients.
Randomised patients received either osilodrostat or placebo. At week 34, the end of an eight-week randomised withdrawal period, 86% of patients treated with osilodrostat showed normal mean urinary free cortisol (mUFC) compared to only 29% of patients receiving placebo. At week 48, 66% of the total patients had normal levels of mUFC.
Another phase III, multi-centre, randomised, double-blind, 48-week clinical study, LINC-4 is being performed in 73 patients to evaluate the safety and efficacy of osilodrostat in patients who are medical therapy candidates.
The most common adverse events observed in patients during the trial were low levels of cortisol, fatigue, nausea, headache, and swelling in legs and ankles or fluid retention in the body.