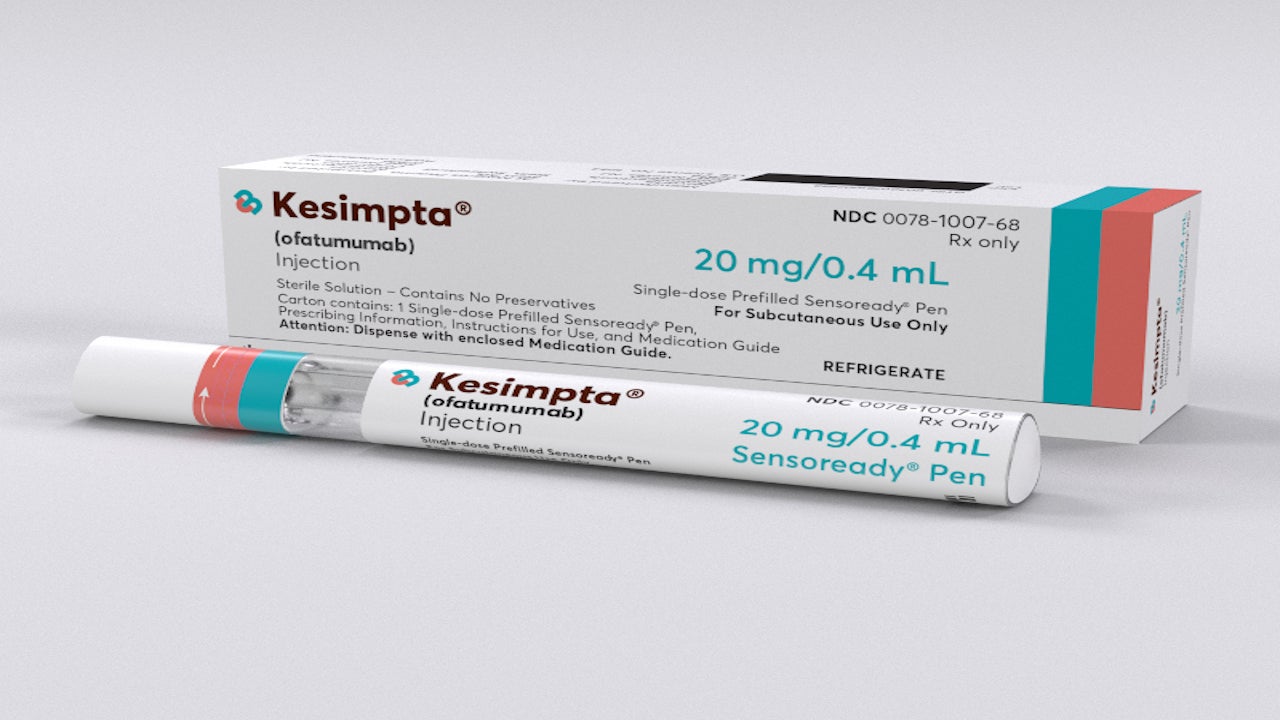
Kesimpta® (ofatumumab) is the first and only self-administered, targeted B-cell therapy indicated for the treatment of patients with relapsing multiple sclerosis (RMS) including clinically isolated syndrome (CIS), relapsing-remitting (RRMS), and active secondary progressive multiple sclerosis (SPMS).
Kesimpta (ofatumumab) is a first-choice, precise dosage treatment option for RMS patients. Ofatumumab was originally developed by GlaxoSmithKline (GSK) and Genmab. GSK transferred its rights of ofatumumab for all indications, including MS, to Novartis Pharma in December 2015. The drug is currently developed and commercialised jointly by Genmab and Novartis Pharma globally.
It is a once-monthly subcutaneous injection available in 20mg / 0.4mL dosage strength delivered, using a single-dose prefilled Sensoready® autoinjector pen or a single-dose prefilled syringe.
Kesimpta approvals
The US Food and Drug Administration (FDA) first approved ofatumumab under the brand name Arzerra™ to treat chronic lymphocytic leukaemia (CLL) patients in October 2009. The approval was extended for the previously-untreated CLL patients in April 2014.
Novartis submitted a supplementary biologics license application (sBLA) for ofatumumab to the FDA for the treatment of RMS in December 2019. The drug received approval under the trade name Kesimpta in February 2020.
The European Medicines Agency (EMA) accepted marketing authorisation application (MAA) for the drug in February 2020, and the regulatory approval is expected in Europe by the second quarter of 2021.
Relapsing multiple sclerosis (RMS) causes and symptoms
Multiple sclerosis (MS) is an auto-immune disease that affects the central nervous system, characterised by the destruction of the myelin sheath surrounding the nerve fibres of the brain, optic nerves, and spinal cord.
MS can be categorised into four main types, clinically isolated syndrome (CIS), relapsing-remitting (RRMS), secondary progressive MS (SPMS), and primary progressive MS (PPMS). Relapsing forms of MS include CIS, RRMS, and active SPMS.
Different types of MS can be distinguished depending on whether a patient suffers relapses (clearly specified acute inflammatory attacks with deteriorating neurological function) or experiences progression with neurological damage and disability from the onset of the disease.
Symptoms include numbness and tingling, eye pain, and vision problems (double or jumpy vision), dizziness, bowel or bladder problems, depression and fatigue.
Ofatumumab mechanism of action
Ofatumumab is an anti-CD20 monoclonal antibody (mAb) whose exact mechanism of action on MS patients is unknown.
The drug is believed to bind to the CD20 cell surface antigen located on the pre-B and mature B lymphocytes, resulting in the antibody-dependent cellular cytolysis and complement-mediated lysis.
Safety or effectiveness of the drug in children is currently unknown.
Clinical trials on Kesimpta
FDA approval of Kesimpta (formerly OMB157) is based on the results of the two Phase III, randomised, double-dummy, double-blind, active comparator-controlled, multi-centre clinical trials, ASCLEPIOS I and II.
Both the identically designed studies enrolled a total of 1,882 MS patients of the age group between 18 and 55 years.
In study 1, 927 patients were randomised to receive 20mg of Kesimpta (N=465) or 14mg of teriflunomide (N=462). In study 2, 955 patients were randomised to receive 20mg Kesimpta (N=481) or 14mg teriflunomide (N=474).
The primary goal of the studies was to evaluate the annualised relapse rate (ARR) in patients, treated for up to 30 months, to establish the superiority of ofatumumab compared to teriflunomide in reducing the frequency of confirmed relapses.
Kesimpta significantly lowered the ARR by 51% and 59% in patients in study 1 and study 2, respectively, compared to teriflunomide.
It significantly reduced the risk of a confirmed disability progression in patients by 34.4% in three months, compared to teriflunomide in both studies.
Kesimpta significantly reduced the number of T1 Gd-enhancing lesions (98% and 94%) and the rate of new or widening T2 lesions per year (82% and 85%) in both studies.
Kesimpta delivers superior efficacy with a safety profile similar to teriflunomide. Once-monthly dosing of ofatumumab allows faster B-cells satiation with more flexibility.
The most common adverse reactions observed in patients during clinical trials were headache, upper respiratory tract infection, local injection site reactions and injection-related reactions.