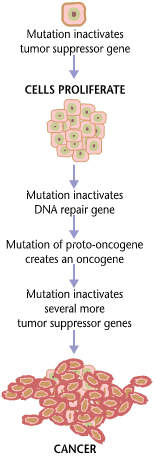
KRX-0401 (perifosine) is a potential oral anti-cancer agent that suppresses the pathways associated with the programmed growth, death, differentiation and survival of cells. The drug, developed by Keryx, is most effective at modulating the Akt, JNK and MAPK pathways.
In clinical trials conducted in the US and Europe, KRX-0401 has been used to treat more than 1,800 cancer patients. It has been used as a single agent and in combination with novel agents.
KRX-0401 is undergoing its Phase III clinical trials for the treatment of refractory multiple myeloma, in combination with a placebo, and is in Phase II trials for several other cancers.
In April 2010, Keryx initiated enrolment for a Phase III clinical trial of KRX-0401 for the treatment of refractory advanced colorectal cancer. The randomised, double blind X-PECT (Xeloda + Perifosine Evaluation in Colorectal Cancer Treatment) trial will be conducted across 50 centres in the US.
The study is being carried out under a special protocol assessment with the US Food and Drug Administration (FDA). About 465 patients have been enrolled in the study, which is scheduled for completion in February 2012.
KRX-0401also received fast-track designation from the FDA in April 2010. This designation hastens the review process of new drugs that are being developed for life threatening diseases. Keryx expects to commence commercialisation of KRX-0401 by mid-2012.
It has also been awarded the orphan-drug designation by the FDA for the treatment of neuroblastoma, a cancer of the nervous system.
Multiple myeloma
Multiple myeloma is an incurable cancer occurring in plasma cells, a type of white blood cell. The disease results from the production of multiple abnormal plasma cells in the bone marrow, leading to tumours in several bones of the body.
These tumours prevent the bone marrow from building sufficient healthy blood cells. When the number of plasma cells continues to increase even after the patient is provided with treatment, the condition is known as refractory multiple myeloma.
The disease is treatable but, despite of the availability of FDA-approved therapies, many patients relapse, become refractory and finally die because of other diseases each year. In 2009, there were around 10,580 of these deaths in the US.
Clinical trials
Keryx launched a combined Phase I/II study on KRX-0401 in combination with bortezomib to evaluate it as a treatment for refractory multiple myeloma.
The study involved 84 patients, of which 83% had refractory multiple myeloma. On average, patients had five of prior lines of therapy, and all were pre-treated with bortezomib-based and bortezomib plus dexamethasone-based therapies.
In addition, 98% of patients had previously been treated with dexamethasone, while 99% had prior treatment with lenalidomide (revlimid and/or thalidomide). About 57% of patients had previously undergone stem cell transplant.
In the Phase I component 18 patients were enrolled. During the trial period, KRX-0401 was increased from 50 mg to 100mg once daily. Bortezomib was given in doses of 1.0-1.3mg/m². There were no incidences of adverse events that prevented the dose from being increased, and toxicities were generally well administered and tolerated. Patients with progressive disease who were on the combination of KRX-0401 and bortezomib were given a 20mg dose of dexamethasone on the day they were administered bortezomib and the day after.
In Phase II, which involved 66 patients, 50mg of KRX-0401 was given once daily along with 1.3mg/ m² of bortezomib. During analysis, responses from 73 patients were evaluated.
Results
The clinical results of the combined Phase I/II study were announced in February 2009. In conjunction with bortezomib and dexamethasone, KRX-0401 exhibited an impressive response rate and time-to-progression. Nearly 83% of the patients who were evaluated were found to have attained stable disease or better.
The overall response rate and time-to-progression of bortezomib-relapsed patients also improved to 55%. Patients in whom the disease relapsed after taking a bortezomib-based treatment showed an average time-to-progression of 8.5 months. The average time-to-progression of all the evaluable patients was 6.4 months.
Encouraged by the results of the Phase I/II study, Keryx had begun the preliminary work for a randomised Phase III trial on the same combination by mid-2009.
Phase III
In August 2009, Keryx agreed a special protocol assessment with the FDA on the design of KRX-0401’s Phase III trial.
Enrolment for this trial began in December 2009. It involves 450 patients suffering from refractory multiple myeloma. Apart from undergoing one to four prior lines of therapy, all the patients will be pre-treated with bortezomib and Revlimid or thalidomid, an immunomodulatory agent.
The trial is double-blind and placebo-controlled. It will assess the efficacy and safety of KRX-0401 in comparison with placebo when both are given with bortezomib and dexamethasone, and is expected to be completed by October 2012.
The clinical endpoints of the trial will primarily include survival without progression. Overall response rate, survival and safety will be the secondary end points of the trail.
Trials for multiple other forms of cancer
KRX-0401 has also been evaluated through clinical trials conducted on patients suffering from several types of cancers. The Phase I trial of KRX-0401 for recurrent paediatric solid tumours began in July 2009 at the Memorial Sloan-Kettering Cancer Center in the US.
In June 2010, clinical data from the Phase I study was presented at the 46th Annual Meeting of the American Society of Clinical Oncology (ASCO) in Chicago. The data indicated that KRX-0401 was well tolerated in children. The data also indicated that the drug can be investigated for advanced neuroblastoma and other brain tumours.
In June 2009, Keryx announced positive results from a randomised Phase II trial of KRX-0401 for the treatment of advanced metastatic colon cancer.
In January 2010, Keryx announced results from a Phase II trial investigating KRX-0401 for the treatment of Waldenström’s macroglobulinemia. The study compared the safety and efficacy of perifosine in combination with capecitabine versus capecitabine and a placebo.
The results indicated that perifosine administered in combination with capecitabine demonstrated a 60% increase in overall survival rate compared to those administered with capecitabine and a placebo.