Lampit® (nifurtimox) is an antiprotozoal medication indicated for the treatment of Chagas disease (American Trypanosomiasis) in paediatric patients from birth to less than 18 years of age and weighing at least 2.5kg.
Developed by Bayer, Lampit is available as yellow round, biconvex, functionally scored tablets in 30mg and 120mg dosage strengths for oral administration. It is specially formulated to disperse the medicine in water to support the delivery in paediatric patients.
Lampit approvals
The new drug application (NDA) for Lampit (nifurtimox) was submitted to the US Food and Drug Administration (FDA) in June 2019.
The drug received accelerated approval from the FDA in August 2020. Lampit also holds orphan drug designation from the FDA.
The drug is registered in several Latin American countries, including Argentina, Uruguay, Chile, El Salvador, Guatemala, and Honduras to treat acute and chronic Chagas disease in adults and children.
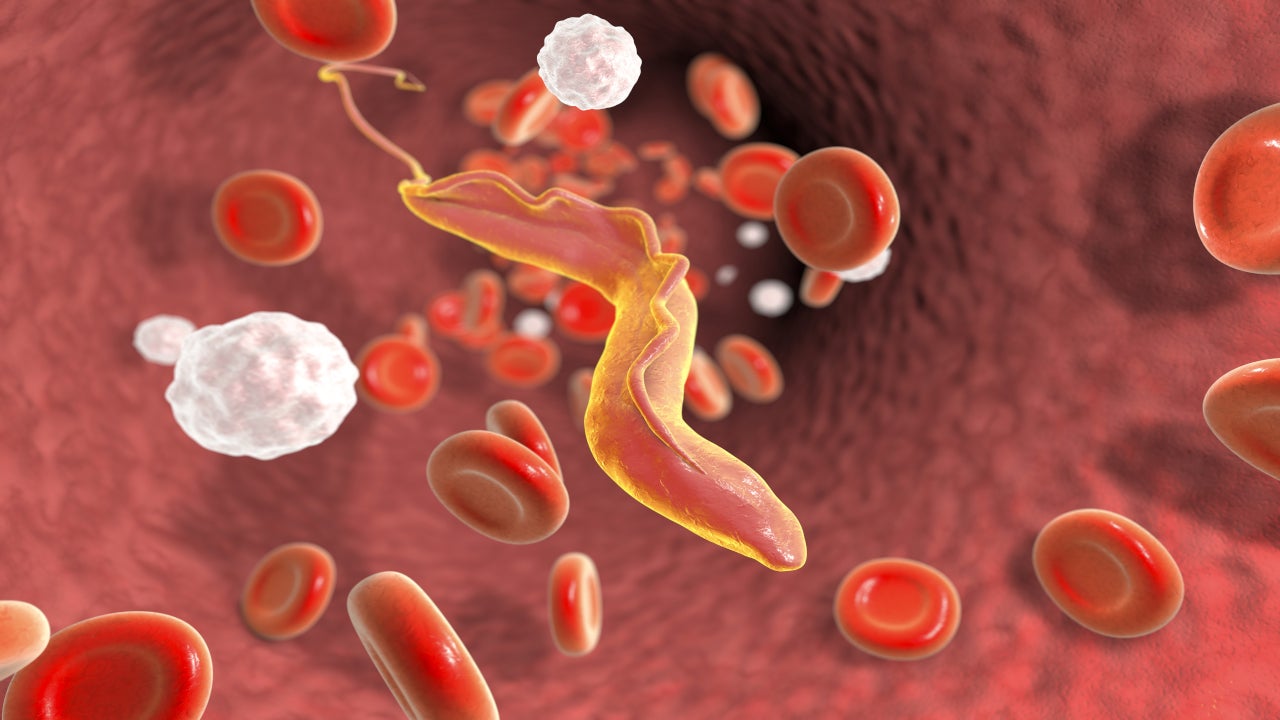
Chagas disease cause and symptoms
Chagas disease is an infectious disease caused by the single-celled Trypanosoma cruzi (T. cruzi) parasite. The disease spreads to humans by the faeces of blood-sucking carrier bugs called triatomines or kissing bugs.
It may also spread through infected organ transplantation, infected blood transfusion, infected mother to her infant during pregnancy or delivery, and less commonly through the consumption of contaminated food.
Chagas disease can be life-threatening in both the acute and chronic stages of infection. Acute infection may be asymptomatic but can also include mild to severe itching or rash or lack of appetite at the insect bite site.
Clinical symptoms can include mild to severe hepatomegaly or lymph node enlargement. Chronic infectious patients can also be asymptomatic. The disease is endemic across Mexico, Central America, and South America and affects more than eight million people worldwide.
The disease is curable if detected and treated soon after infection. Untreated people can move to the chronic phase of the disease, and approximately 20% to 30% of patients with chronic infections suffer from gastrointestinal or cardiac complications.
Nifurtimox mechanism of action
Nifurtimox is a nitrofuran derivative and acts as an antiprotozoal drug. It is activated by type I (oxygen insensitive) and type II (oxygen-sensitive) nitroreductase (NTR), resulting in the production of toxic intermediate metabolites or reactive oxygen species that trigger DNA damage and cell death of both intracellular and extracellular forms of T. cruzi.
Nifurtimox is active against all three stages of T. cruzi, namely trypomastigotes, amastigotes, and epimastigotes.
The potential for developing resistance against nifurtimox in T. cruzi is indicated in in-vitro studies.
It is unknown if Lampit is safe and effective in children weighing less than 2.5kg.
Clinical trials on Lampit
The FDA approval of Lampit was based on the results of a randomised, double-blind, prospective, phase three clinical trial. The trial was conducted at 25 investigating sites in Colombia, Bolivia, and Argentina.
The trial evaluated the safety and efficacy of Lampit in 330 paediatric patients aged less than 18 years and weighing at least 2.5kg.
Patients were randomised at a 2:1 ratio to receive either a 60-day (n=219) or 30-day (n=111) nifurtimox treatment and were followed up for one year.
Patients in the nifurtimox 60-day arm showed superior results compared to the nifurtimox 30-day arm. The serological response, using lysate ELISA in the 60-day arm was 32% compared to 19% in the 30-day arm, while the primary efficacy endpoint for the recombinant ELISA was 35% in the nifurtimox 60-day arm and 22% in the 30-day arm.
The common side effects observed in patients during the clinical trial were abdominal pain, vomiting, diarrhoea, decreased appetite, nausea, anaemia, eosinophilia, decreased weight, urticaria, dizziness, pyrexia, rash, and headache.