Lipitor (Atorvastatin) is a statin class drug indicated for reducing high cholesterol levels in the blood, which helps in the prevention of cardiovascular diseases. It is manufactured and marketed by Pfizer. Pfizer received the US Food and Drug Administration (FDA) approval for lipitor in 1996.
Patent protection on lipitor in the US expired in November 2011. Atorvastatin, the generic version of lipitor, was approved by the FDA in November 2011. Generic lipitor is being manufactured by Watson Laboratories and Ranbaxy.
Ranbaxy also introduced the generic version of the drug in Italy, the Netherlands and Sweden before its scheduled patent expiry in May 2012 in these countries.
Cholesterol levels and links to cardiovascular diseases
Cholesterol is a combination of fat and steroid, a fat-soluble organic compound which is produced naturally in the human body. About 80% of the cholesterol is produced in the liver, while the rest of it comes from foods such as fish, meat and milk.
Cholesterol is of two types, namely low density lipoprotein (LDL) cholesterol and high density lipoprotein (HDL) cholesterol. LDL is associated with cardiovascular diseases, whereas HDL helps in preventing thickening of artery (atherosclerosis).
A high level of cholesterol in blood is known as hyperlipidemia, which causes the build-up of walls in arteries and can lead to the risk of getting cardiovascular diseases.
Lipitor’s mechanism of action and doses available
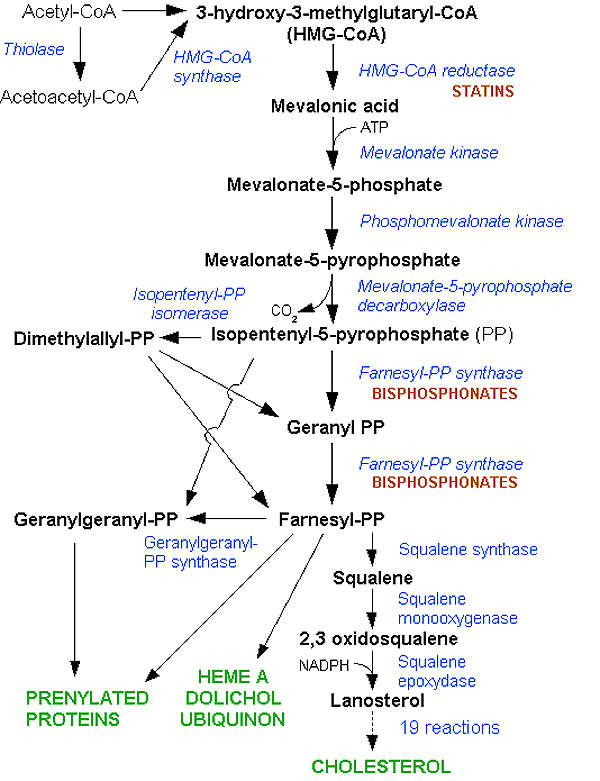
Statin present in lipitor (atorvastatin) lowers the cholesterol in the body by blocking the excess cholesterol-producing enzyme in the liver. The drug works on inhibiting HMG-coenzyme A reductase (HMG-CoA) and decreases the production of LDL cholesterol.
The drug reduces the risk of cardiovascular diseases by lowering the excess cholesterol in the blood vessels leading to the brain. The drug is available in ten, 20, 40 and 80mg doses.
Phase III clinical trials on Pfizer’s lipitor
Lipitor was approved by the FDA, based on the results of four Phase III clinical trials.
The first Phase III clinical trial, known as the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), was conducted on 10,305 patients aged between 40 and 80 years-old. It was a double-blind, placebo-controlled study which evaluated the effectiveness of lipitor on fatal and non-fatal coronary heart diseases.
Results of the study showed that the drug significantly reduced fatal coronary heart disease. It reduced non-fatal myocardial infarction (MI) events to 60 when compared to 108 in placebo patients. The drug also decreased the revascularization procedures by 42%.
The second Phase III clinical trial, the Collaborative Atorvastatin Diabetes Study (CARDS), was a multicentre, double-blind and placebo-controlled study. It evaluated the effectiveness of lipitor on cardiovascular diseases. The study enrolled 2,838 patients aged between 40 and 75 years of age.
The results showed the drug reduced the rate of cardiovascular events to 83 when compared to 127 in the placebo group. It reduced the risk of stroke by 48% and risk of MI by 42%.
The third Phase III clinical trial on lipitor was named Treating to New Targets (TNT) study. It evaluated the effectiveness of 10mg in comparison with 80mg doses of the drug. It enrolled 10,001 subjects. The primary endpoint of the study was finding the major cardiovascular events.
The drug reduced events in the 80mg dose group to 434 when compared to 548 events in the 10mg group.
The fourth Phase III clinical trial on lipitor, called the Incremental Decrease in Endpoints Through Aggressive Lipid Lowering (IDEAL) study, enrolled 8,888 patients. It evaluated lipitor 80mg doses with simvastatin 20mg to 40mg doses.
The results of the study showed that the rate of major coronary events in the 80mg lipitor group was reduced to 411, compared to 463 in the simvastatin group. Lipitor 80mg doses were thus found to be more effective than simvastatin 20mg to 40mg doses.
Marketing commentary for lipitor and its generic competitors
Ranbaxy launched the generic version of lipitor in the US market in November 2011. Ranbaxy shares the profit on generic sales of the drug in the first six months with Israel-based Teva Pharmaceutical Industries (TEVA).
Launch of atorvastatin has led to price war between Pfizer and other generic drug makers. In December 2011, Pfizer slashed the price of lipitor by 80% of its original cost. The sales of the drug in the US reached $13bn in 2011.