Myrbetriq (mirabegron) is a beta-3 adrenergic agonist indicated for the treatment of the overactive bladder (OAB) condition. It was developed by Japan’s Tokyo-based pharmaceutical company, Astellas Pharma.
The drug was approved by the US Food and Drug Administration (FDA) in June 2012, for treating the patients with OAB. The drug was approved in Japan in July 2011.
Myrbetriq is the first oral treatment for OAB. It was launched in US pharmacies in October 2012.
The drug is available in 25mg and 50mg doses. It is also under the regulatory review process in several other countries.
Overactive bladder (OAB) condition cause and symptoms
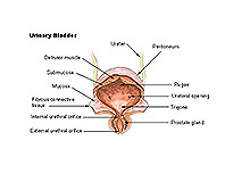
Overactive bladder (OAB) is a urological condition which occurs as a result of sudden and involuntary contraction of wall muscle in the urinary bladder.
OAB is referred to as urge incontinence, which leads to an unstoppable and sudden need to urinate, even when there is a small amount of urine contained in the bladder.
The US FDA’s Centre for Drug Evaluation and Research (CDER) estimates that more than 33 million people in the US suffer from the overactive bladder condition.
Active ingredients and mechanism of action of myrbetriq
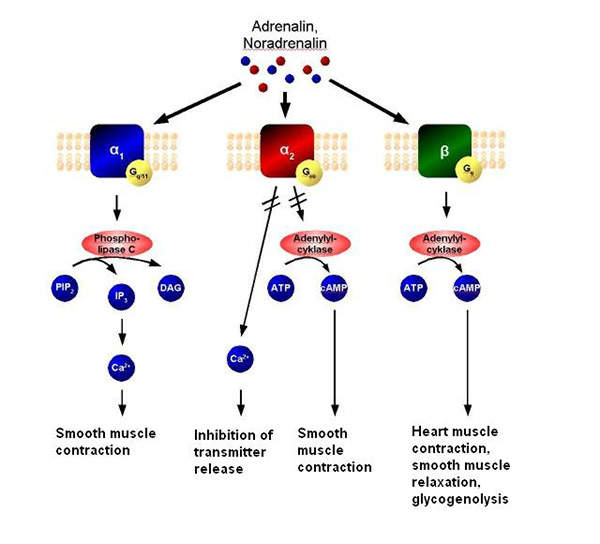
Myrbetriq contains beta-3 adrenergic agonist which increases bladder capacity. The drug contains an anticholinergic agent, which is a substance that blocks the neurotransmitter acetylcholine in the central and the peripheral nervous system and stops involuntary bladder contractions. The drug also relaxes the detrusor urinae muscle during the storage phase.
Clinical trials on mirabegron for treating OAB
Astellas Pharma conducted Phase I clinical trials on myrbetriq between April 2009 and June 2009. It was an open label, non-randomised, pharmacokinetics study. It enrolled 40 patients.
Related project
Votrient – Treatment for Renal Cell Cancer and Soft Tissue Sarcoma, US
GlaxoSmithKline’s (GSK’s) Votrient is an approved medication for the treatment of renal cell cancer (RCC).
The primary outcome measure of the study was the assessment of the pharmacokinetics of mirabegron in comparison with solifenacin through analysis of blood samples. Solifenacin is another OAB drug manufactured by Astellas.
The company conducted Phase II clinical trials on myrbetriq from March 2011 to June 2012. It was a randomised, double-blind, placebo controlled, parallel group and multicentre clinical study. It enrolled 2,101 patients with overactive bladder conditions.
The primary outcome measure of the study was finding the change from the baseline in mean volume voided per micturition. The secondary outcome measures included finding the change in baseline in mean number of incontinence episodes in a day and finding the change in baseline mean number of urgency incontinence episodes in a 24 hour period.
Astellas Pharma conducted Phase III clinical trials on myrbetriq between April 2008 and May 2010. It was a randomised, double-blind, parallel group, multicentre safety study which evaluated the efficacy of the beta-3 agonist in subjects with symptoms of OAB. The study enrolled 2,452 patients.
The primary outcome measure of the study was assessment of incidence and severity of treatment emerged adverse events. The secondary outcome measures included finding the change in mean number of micturitions from the baseline and the change in mean number of incontinence episodes in 24 hours.
The FDA approval for myrbetriq was based on three Phase III clinical trials. The studies enrolled more than 2,700 patients in 12-week, double-blind, placebo-controlled studies. The studies were conducted on patients who consisted of 94% Caucasian and 72% female, with a mean age of 59 years.
Results of the study on Astellas Pharma’s drug
The results of the studies showed that the patients administered with myrbetriq 25mg and 50mg doses improved significantly in efficacy parameters of incontinence episodes and number of urinations in 24 hours.
The incontinence episodes were reduced by 1.36 episodes from a baseline of 2.65 in patients treated with myrbetriq 25mg, when compared to placebo, after 12 weeks.
Myrbetriq 25mg also reduced the number of urinations by 1.65 from the baseline of 11.68. The patients were compared with myrbetriq 50mg and placebo. The number of incontinence episodes in myrbetriq 50mg group was reduced by 1.49, from the baseline of 2.71, and the number of urinations in myrbetriq 50mg group was also reduced by 1.75 urinations, from baseline of 11.70, also after a 12 week period.
The adverse reactions reported in the studies included headache, nasopharyngitis, hypertension and urinary tract infection.