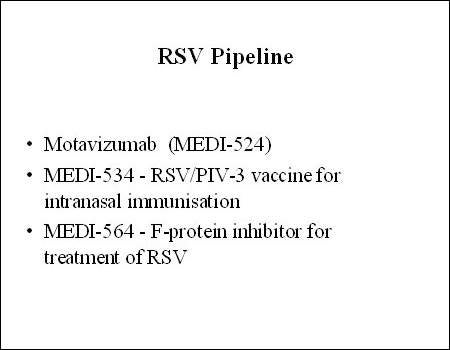
Developed by MedImmune (now wholly owned by AstraZeneca), motavizumab is an investigational monoclonal antibody (MAb) indicated for the prevention of respiratory syncytial virus (RSV) infection in high-risk infants.
Following the successful outcome of large-scale Phase III paediatric clinical trials, a Biologics License Application (BLA) was submitted to the US Food and Drug Administration (FDA) in early 2008 to seek approval to market motavizumab for RSV prevention.
Clinical trial information based on an assessment of the safety and efficacy in preventing RSV in high-risk patients has been gathered from over 6,000 paediatric patients. This data functions as a backbone for the BLA.
The commercialisation of motavizumab in the US will be performed by MedImmune using its marketing and sales organisations. Once the drug receives authorisation for marketing from regulatory bodies in markets outside of the US, MedImmune will promote the product in conjunction with Abbott. Abbott has marketing and distribution rights for the drug in non-US markets.
In reply to the BLA submitted by MedImmune, the FDA issued a Complete Response Letter in November 2008 to the company requesting more information on motavizumab.
In December 2009, AstraZeneca replied to the Complete Response Letter issued by the FDA. AstraZeneca mentioned that additional clinical trials were not required to be conducted. The company also said that it will continue to have discussions with the FDA, when needed, during the registration process of motavizumab. Analysts speculate that the approval of the drug will be delayed by another 18 months due to these developments.
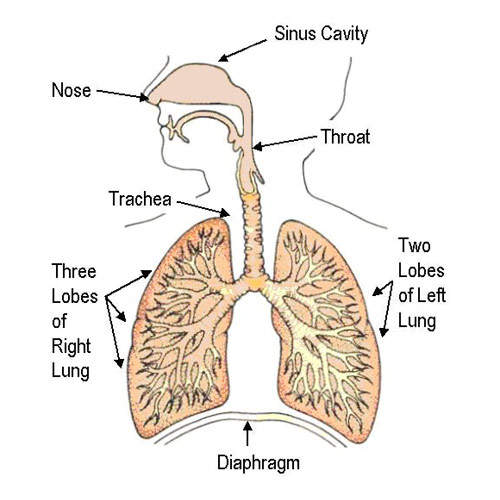
RSV – a serious pulmonary infection
RSV is among the most common respiratory tract infections in childhood and the most common cause of bronchiolitis and pneumonia in infants and children under a year old. Between 25% and 40% of infants and young children experiencing their first RSV infection have signs or symptoms of bronchiolitis or pneumonia, and up to 2% require hospitalisation.
Those most at risk of serious pulmonary disease include pre-term infants as well as those with chronic lung disease and congenital heart disease (CHD).
RSV can also cause repeated infections throughout life and may lead to severe lower respiratory tract disease among the elderly, as well as those whose cardiac, pulmonary, or immune systems are compromised.
Building on the success of Synagis
Ideally, infection with RSV would be prevented by vaccination. Although research to this end is ongoing, no vaccine is yet commercially available. Current preventive strategies for RSV infection include the use of MedImmune’s Synagis (palivizumab), a second-generation humanised MAb against the F-protein of RSV. By binding to this protein, Synagis stops RSV from gaining access to lung cells. By preventing cell-to-cell spreading, it stops the formation of syncytia in the lungs.
Synagis is licensed by the FDA for RSV prevention in high-risk infants, children with chronic lung disease or history of premature birth (≤ 35 weeks gestation) and children with haemodynamically significant CHD. It is usually administered just before the onset of the RSV season and then given monthly while there is still a risk of infection. In the northern hemisphere, the RSV season runs from October to the following April.
The development of motavizumab is part of MedImmune’s ongoing research programme in RSV prevention, which began with the introduction of RSV immune globulin (RespiGam), the first anti-RSV product, and then Synagis.
Like Synagis, motavizumab is a humanised MAb against the F-protein of RSV but with enhanced binding that results in greater neutralising activity. Preclinical studies have shown that it effectively suppresses RSV replication with resultant improvement in disease severity.
Clinical data support efficacy and safety
Clinical trials have compared the efficacy and safety of motavizumab with both a placebo and Synagis (standard care).
Results from the Phase III trial, comprising over 1,400 high-risk paediatric patients, in which motavizumab was compared with a placebo showed a significantly greater reduction in hospitalisations due to RSV (primary endpoint) in the motavizumab treatment arm compared with a placebo (1.4 vs. 8.3% respectively, p<0.001).
Significantly fewer infants in the motavizumab treatment arm experienced RSV-related lower respiratory infection that required outpatient management.
In the active-controlled Phase III trial in which motavizumab was compared head-to-head with Synagis, it proved to be at least, if not more, as effective than its predecessor. In this, the first pivotal trial, motavizumab demonstrated a 26% reduction in RSV-associated hospitalisation and a 50% reduction in the incidence of RSV lower respiratory tract infections requiring outpatient management, its secondary endpoint.
Motavizumab was well tolerated by paediatric patients enrolled in these trials with adverse events consistent with previous experience with Synagis.
Observational study to evaluate RSV burden
To evaluate RSV burden in premature babies that come under the 32–35-week gestational age (GA) group in an outpatient environment within the first year of birth, MedImmune is performing an initial two-year observational prospective study. The study will research two successive seasons of RSV and identify the reasons that might increase the incidence of RSV.
The study will endeavour to recognise the premature infants most likely to contract the infection and will feature virology information on the RSV season, spanning four geographic areas set up by the US Centers for Disease Control & Prevention.
Nearly 3,000 participants will take part in the study that will span 100 US outpatient sites. Premature babies in the 32–35-week GA group who have not been administered RSV prophylaxis in their initial RSV season will also be part of the study population. The enrolment of patients will begin by late 2009.
Marketing commentary
Approval of MedImmune’s Synagis (palivizumab) in 1998 marked an important advance in the management of RSV-associated bronchiolitis and pneumonia in high-risk infants. It continues to occupy a niche position in the anti-infectives market as the only licensed MAb for RSV prevention.
A potential successor to Synagis, motavizumab may provide even more effective prophylaxis in at-risk groups. Longer term, MedImmune is exploring the potential of a small-molecule drug for RSV prevention and a vaccine against RSV.