Onpattro™ (patisaran) is the first-of-its-kind RNA interference (RNAi)-based drug indicated for the treatment of polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults.
Alnylam Pharmaceuticals, a biopharma company based in the US, developed the drug. In December 2017, the company submitted a new drug application for patisiran to the US Food and Drug Administration (FDA), which was approved in August 2018 as the first drug for the treatment of polyneuropathy of hATTR.
Alnylam submitted the marketing authorisation application (MAA) for patisiran to the European Medicines Agency (EMA) in December 2017. The drug was approved by the European Commission (EC) in August 2018, also receiving approval in Japan in June 2019 and Canada in July 2019.
Alnylam signed an agreement with Genesis Pharma in July 2019 to commercialise patisiran in 12 countries including Serbia, Bosnia and Herzegovina, Romania, Slovenia, Croatia, Albania, Republic of North Macedonia, Montenegro, Malta, Greece, Cyprus and Bulgaria.
The company filed a marketing authorisation application with the Brazilian Health Regulatory Agency (ANVISA) for patisiran in Brazil in October 2019 and received priority review status. The decision from ANVISA is expected in the first six months of 2020.
Hereditary ATTR disease causes and symptoms
Hereditary ATTR is a rare and progressive genetic disease caused by the misfolding or deformation of a protein called transthyretin (TTR), formed in the liver. The condition leads to the accumulation of amyloid deposits in various parts of the body, including heart, nerve and gastrointestinal tract.
Some common symptoms include numbness, pain and weakness in limbs, cardiovascular issues, diarrhoea, nausea, vomiting and kidney dysfunction. The disease is estimated to affect approximately 50,000 patients globally.
Onpattro’s mechanism of action
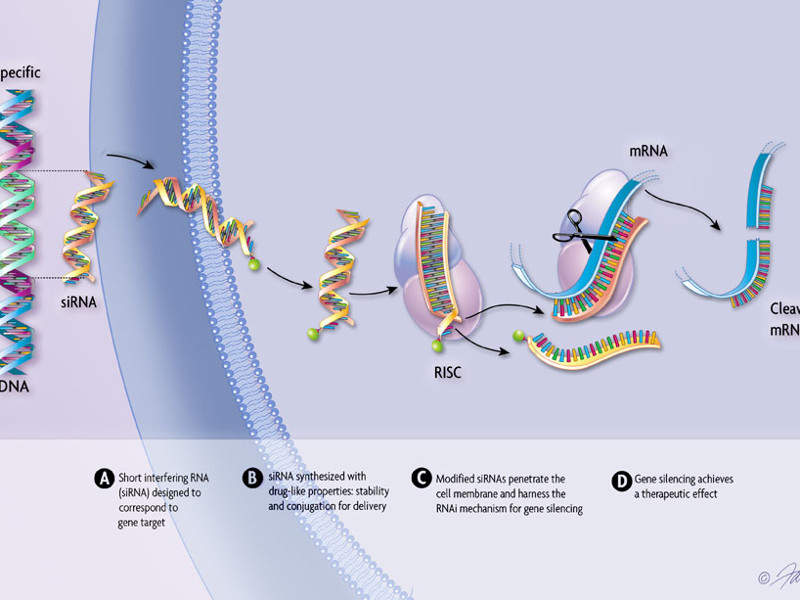
Onpattro contains patisaran, a double-stranded small interfering ribonucleic acid (siRNA) formulated in the form of a lipid complex. The drug binds to the TTR protein and prevents its deformation through the RNA interference. It helps in reducing the level of TTR protein made in the liver and the resultant amyloid deposits in the tissues.
Onpattro lipid complex injection is available as white to off-white homogeneous formulation in a single-dose vial in 10mg per 5mL (2mg per mL) strength.
Clinical trials on Onpattro
FDA approval of Onpattro comes from positive results of a global phase three clinical trial, APOLLO.
The APOLLO trial was a randomised, double-blind, placebo-controlled, clinical study performed to evaluate the safety and efficacy of the drug in patients. A total of 225 patients were enrolled for the trial and randomised to Onpattro and placebo in a 2:1 ratio for 18 months.
Out of the total 225 patients, 148 received Onpattro (0.3mg per kg of body weight) once in every three weeks and the remaining 77 patients received the placebo. The primary endpoint of the study was a modification in the Neuropathy Impairment Score +7 (mNIS+7) from baseline at 18 months. The score was used to assess the improvement in motor skills, nerve conduction and postural blood pressure of the patients.
Patients receiving intravenous Onpattro infusion demonstrated measurable improvements in symptoms including polyneuropathy, daily activity performance, quality of life and ambulation nutritional status.
Further, 51% of the patients treated with Onpattro showed improvement in the quality of life measured by the Norfolk Quality of Life Diabetic Neuropathy (QoL-DN) compared to 10% of those treated with placebo.
The most common adverse events reported in the trial were upper respiratory tract infections and infusion-related reactions, while back pain, abdominal pain and headache were some of the symptoms associated with infusion-related reactions (IRRs).
Marketing commentary on Alnylam
Headquartered in Massachusetts, US, Alnylam is a biopharmaceutical company focused on the development of innovative RNAi-based therapeutics to treat various diseases.
The company’s pipeline contains innovative investigational therapeutics under broad areas such as genetic medicines, cardio-metabolic diseases, hepatic infectious diseases and central nervous system diseases.
Some late-stage pipeline products of the company include givosiran, fitusiran and inclisiran.