Developed by Sanofi-Aventis (formerly Sanofi-Synthelabo), osanetant (SR-142801) is an NK3 receptor antagonist which was under development for the treatment of schizophrenia and other Central Nervous System (CNS) disorders. In a review of its R&D portfolio, the company announced in August 2005 that it would cease any further development of osanetant. This follows an earlier decision to discontinue development of eplivanserin for schizophrenia.
ANTIPSYCHOTIC MEDICINES FOR SCHIZOPHRENIA
Affecting around 1% of the general population, schizophrenia is a severe and debilitating mental illness. Antipsychotic drugs are the cornerstone of the management of schizophrenia and are designed to address the core symptoms of the illness. These include:
- Positive symptoms: hallucinations, delusions, agitation, disorganised thinking
- Negative symptoms: introversion, apathy, low self-esteem leading to personal neglect and more rarely catatonia
- Cognitive symptoms: poor memory, attention deficit, executive dysfunction
- Affective symptoms: depression , elation, suicidal ideation
Despite an expanding range of antipsychotic drugs, which have greatly improved treatment prospects for patients with schizophrenia, there remain significant treatment gaps. New antipsychotic drugs are needed with broader efficacy to treat patients refractory to current agents. Although the atypical antipsychotics, which comprise at least 90% of the market, offer improved safety and tolerability to conventional typical antipsychotics safety is still an issue. Significant weight gain leading to metabolic disturbances (diabetes, heart disease), sexual dysfunction, hyperprolactinaemia and cardiotoxicity are common side effects associated with some of the atypical drugs. Poor tolerability leads to poor treatment compliance and risk of relapse. Thus, there is a need for antipsychotics with improved safety and tolerability to improve compliance and patient well-being.
NEUROKININS AS CNS AGENTS
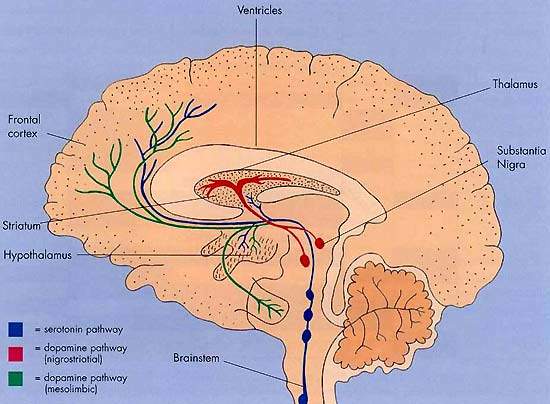
First identified in the 1930s, neurokins are neurotransmitters found in the substantia nigra and striatum areas of the brain. Unlike most of the neurotransmitters identified to date, they are made from peptides rather than amino acids. They are and believed to be involved in the control of movement. Their potential as therapeutic targets for drug development has only recently been realised but is seen an area of rich research. The neurokinins NK1 and NK3 have been identified as suitable targets for drug development. Several antagonists to these neurokinins are now in development. With GSK’s talnetant, osanetant was one of two NK3 antagonists in development for schizophrenia.
OSANETANT ANTIPSYCHOTIC ACTIVITY IDENTIFIED VIA METATRIAL PROTOCOL
The potential antipsychotic properties of osanetant were identified in a special study protocol termed a Metatrial. The objective was to test concomitantly for both safety and efficacy four compounds which were judged to have antipsychotic properties based on their pharmacological profiles. This study design had the advantage of decreasing patient numbers in the placebo and calibrator (haloperidol, 10mg/day) arms of the study. Four compounds were tested.
Results showed that while two compounds had no demonstrable clinical efficacy, two showed activity against core symptoms of schizophrenia (osanetant and eplivanserin). Osanetant had an activity and efficacy profile similar to that of haloperidol (a typical antipsychotic) and appeared well tolerated. In contrast to the positive findings in schizophrenia, the results of a study of osanetant in severe depression proved inconclusive. Comparisons between placebo and standard antidepressants, including Selective Serotonin Re-uptake Inhibitors (SSRI), showed no significant difference after six weeks of treatment.
In contrast to the positive findings in schizophrenia, the results of a study of osanetant in severe depression proved inconclusive. Comparisons between placebo and standard antidepressants, including selective serotonin re-uptake inhibitors (SSRIs), showed no significant difference after six weeks of treatment.
MARKETING COMMENTARY
Currently available atypical antispychotic agents target either dopamine or serotinin receptors or both in the brain. Dopamine D2 receptor antagonism is believed to be the primary pharmacological mechanism by which current antipsychotic drugs ameliorate positive psychotic symptoms. The fact that not all patients respond to existing antipsychotic drugs suggests other neurotransmitters may be involved in the pathogenesis of schizophrenia. Neurokinin receptors are just one of several new avenues of research. Others include muscarinic, cannabinoid, kainate, and sigma receptors.
A dynamic sector of the CNS market, the market for antipsychotic drugs is predicted to continue its robust growth. This is driven primarily be the need for safer and more effective antipsychotic drugs. The NK3 antagonists represent an entirely new class of antipsychotic drugs and the decision to cease further development of osanetant raises questions about the viability of this new class of drugs as a treatment for schizophrenia.