Palynziq™ (pegvaliase-pqpz) is a subcutaneous infusion indicated for the treatment of phenylketonuria (PKU).
The drug was developed by BioMarin, a pharmaceutical company based in the US.
A biologics license application (BLA) for the drug was submitted to the US Food and Drug Administration (FDA) in June 2017, and was approved in May 2018. It was one of the first enzyme substitution therapies to be granted marketing authorisation by the FDA for PKU.
The company has also submitted a regulatory filing with the European Medicines Agency (EMA) for marketing approval, which was accepted for review in March 2018 and is currently awaiting approval.
Phenylketonuria disease details
Phenylketonuria is a genetic disorder caused by phenylalanine hydroxylase (PAH) enzyme deficiency. It is estimated to affect more than 50,000 people worldwide.
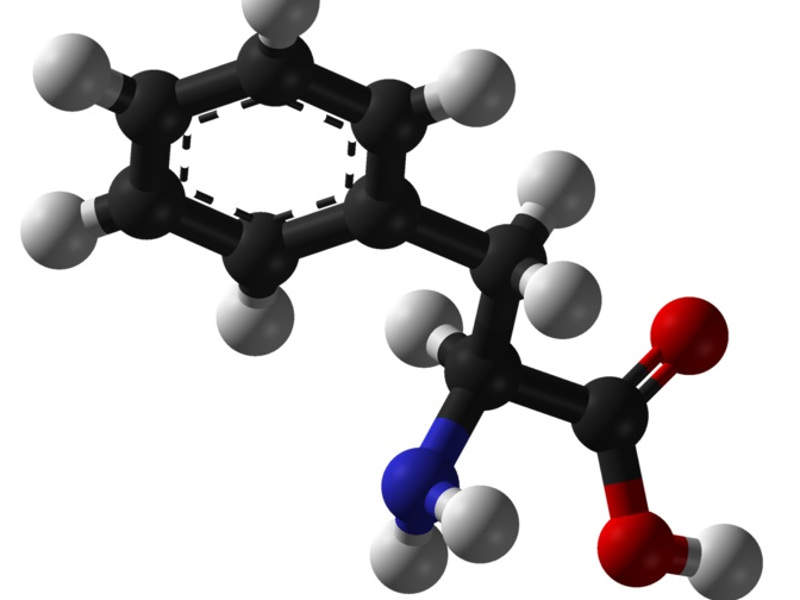
The PAH enzyme reduces blood concentrations of phenylalanine, an amino acid found in all forms of protein. A high level of phenylalanine in the blood is toxic to the brain and can lead to neurological and neuropsychiatric disorders such as intellectual disability, seizures, terrors and psychiatric symptoms.
The most severe form of PKU disorder is known as classic PKU. Infants with classic PKU gradually develop permanent intellectual disability if not treated in a timely manner. Less severe forms of PKU include variant PKU and non-PKU hyperphenylalaninemia, which are associated with minor brain damage.
PKU can be controlled with a phenylalanine-restricted diet and supplements of low-protein modified foods and phenylalanine-free medical foods.
Palynziq’s mechanism of action
Palynziq™ is a PEGylated recombinant phenylalanine ammonia lyase (PAL) enzyme that acts as a substitute for PAH deficiency in PKU patients.
The pegvaliase-pqpz contained in Palynziq™ breaks down the phenylalanine and reduces the blood concentration to normal levels.
Clinical trials on Palynziq
The FDA’s approval for Palynziq™ was based on data from two Phase III trials known as PRISM-1 (NCT01819727) and PRISM-2 (NCT01889862), which evaluated changes in blood phenylalanine concentrations.
The clinical trials were conducted at 60 US sites and involved the participation of more than 285 adult patients with PKU.
The PRISM-1 study was an open-label, randomised, multi-centre study conducted to evaluate the efficacy of Palynziq™ in PKU patients aged 18 to 70 years. The patients were randomised in a 1:1 ratio to either 20mg or 40mg of Palynziq™ once daily or placebo for four weeks. The primary endpoint was achieved with the reduction of blood phenylalanine concentrations.
The PRISM-2 trial was a double-blind, randomised withdrawal period study conducted on PKU patients that had blood phenylalanine levels of more than 600µmol/l on existing management treatments. The study met its primary endpoint by showing lower levels of blood phenylalanine concentrations when compared to patients who received placebo treatment.
More than 57% of patients were taking medical food at baseline, while 16% were on a protein-restricted food at baseline during the Phase III clinical study programme.
The most common adverse reactions of the drug were severe allergic reactions. Arthralgia, hypersensitivity reactions and headaches were also reported as the common side effects of using Palynziq™.
Marketing commentary on BioMarin
BioMarin is a biopharmaceutical company headquartered in California, US.
The company is engaged in the development of therapeutics for patients living with life-threatening rare genetic diseases.
BioMarin’s product portfolio includes seven marketed products and several clinical and pre-clinical drug candidates.
The company’s approved product portfolio includes Palyzinq™, Brineura, Naglazyme, Aldurazyme, Kuvan, Firdapse and Vimizim.