Promacta (eltrombopag) is the first-in-class treatment indicated for patients with severe aplastic anaemia (SAA) immunosuppressive therapy (IST) was proven ineffective for. It was discovered and developed by GlaxoSmithKline (GSK) in partnership with Ligand Pharmaceuticals.
GSK submitted supplemental New Drug Application (sNDA) for Promacta to US Food and Drug Administration (FDA) in February 2014.
The drug was given breakthrough therapy designation by the FDA in January 2014 and was accepted for priority review in April 2014. GSK received approval for Promacta as a once-daily treatment for patients with SAA who were unresponsive to immunosuppressive therapy, in August 2014.
Severe aplastic anaemia (SAA)
An erythropoiesis-stimulating agent (ESA) developed for the treatment of anaemia associated with chronic kidney disease (CKD).
Severe aplastic anaemia is a rare and life-threatening blood condition in which bone marrow is unable to make sufficient red blood cells, white blood cells and blood platelets.
The exact cause of SAA is unknown, but it is believed that the disease is caused due to the body’s autoimmune reaction where the body itself destroys the blood producing stem cells in the bone marrow resulting in insufficient red blood cells.
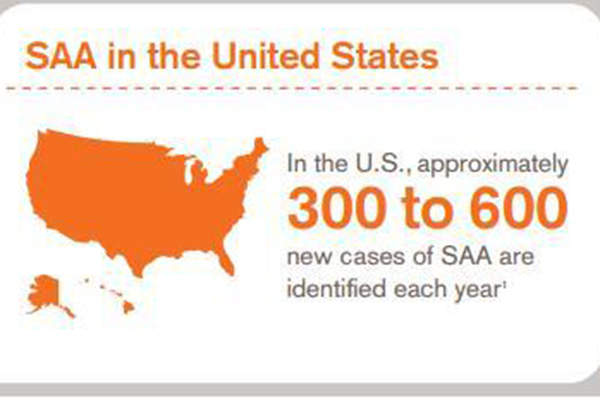
The disease can cause life-threatening infections or bleeding. It is estimated that about 300 to 600 new SAA cases are recorded every year in the US. Immunosuppressive therapy (IST) and hematopoietic stem cell transplantation, which focus on increasing the blood cell count in the patients, are the treatments available for SAA.
Promacta’s mechanism of action
Promacta consists of an active substance called eltrombopag, an oral thrombopoietin (TPO) receptor agonist that helps in increasing the production of blood cells by inducing the proliferation of bone marrow stem cells.
Promacta is available in the form of tablets for oral administration. A 50mg tablet daily once on an empty stomach is the recommended dose of the drug.
Clinical trials on Promacta
The FDA approval for Promacta was based on the results obtained from the phase II clinical trial (09-H-0154) conducted by the National Heart, Lung and Blood Institute (NHLBI) at the National Institutes of Health (NIH).
The phase II study was a single-arm, single-centre, open-label trial. It was conducted on 43 SAA patients who were unresponsive to at least one prior IST. The age group of the patients enrolled for the study was between 17 and 77 years, averaging 45 years, and 56% of them were male.
Patients were administered with an initial dose of Eltrombopag 50mg once daily for two weeks. The dose was slightly increased up to a maximum of 150mg over the following two weeks.
The primary end point of the study assessed after 12 weeks of treatment was the haematologic response observed in 40% patients. Eight patients experienced a multi-lineage response in the extension phase. The treatment was discontinued if no haematologic response was observed.
The study results demonstrated that about 42% of the patients treated with Promacta experienced a hematologic response in at least one lineage after Week 12. It also found that 92% of the patients were platelet transfusion-dependent at baseline, while 86% were RBC-transfusion dependent at baseline.
The most common adverse effects observed during the phase II study in Promacta-administered patients included fatigue, nausea, cough, diarrhoea and headache.
GSK also initiated a Phase III study known as PETIT2 to evaluate the safety and efficacy of Promacta. The study evaluated the drug in comparison with placebo in paediatric patients with chronic immune (idiopathic) thrombocytopenic purpura (cITP).
The primary end point of the study was significant improvement in platelet counts of approximately 40% of the patients treated with eltrombopag. The subjects showed a consistent improvement for six to eight weeks compared to those treated with placebo. The safety profile of the drug was also consistent, with no new safety concerns.
The most common adverse reactions observed in Promacta-administered patients during the phase III study were nasopharyngitis, rhinitis, cough and respiratory tract infection.
Marketing commentary
Eltrombopag is marketed in the US under the brand name Promacta. The drug is marketed as Revolade in other countries.
Eltrombopag is also approved for the treatment of thrombocytopenia in patients with chronic immune (idiopathic) thrombocytopenic purpura (ITP) in 100 countries, and for the treatment of thrombocytopenia (low blood platelet counts) in patients with chronic hepatitis C in 43 countries.