PSI-7977 is indicated for the treatment of chronic Hepatitis C-virus (HCV) infection. The investigational drug is being developed by Pharmasset.
The US Food and Drug Administration (FDA) granted fast track designation to PSI-7977 in August 2010.
Pharmasset is currently conducting Phase IIb clinical trials on the drug to treat HCV-infected people with genotype 1, 2 and 3.
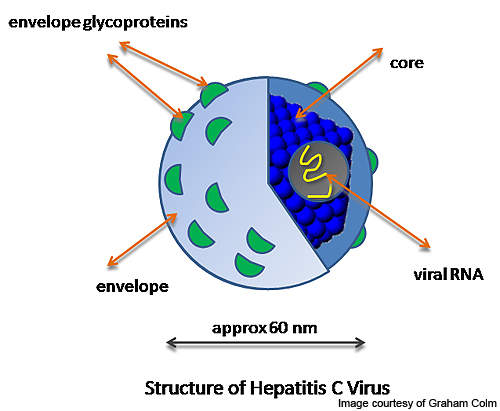
Hepatitis C
Hepatitis C is a contagious disease of the liver, caused by HCV. HCV infection interferes with the human immune system and could result in liver inflammation. Prolonged liver inflammation may cause scarring or cirrhosis, a fatal liver disease.
According to WHO estimates there are approximately 180m HCV-infected people in the world. In the US alone, 3m people are chronically infected with HCV.
Hepatitis C is a human disease that spreads through blood-to-blood contact.
PSI-7977
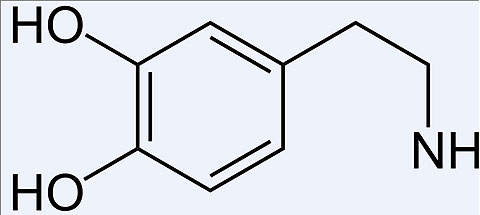
PSI-7977 is a nucleotide analog polymerase inhibitor.
The drug contains a blend of PSI-7976 and PSI-7977 molecules with a similar chemical composition. When the drug enters into the liver cell, both PSI-7976 and PSI-7977 molecules rapidly get converted into the same active triphosphate.
The drug, in a superior form of tablet, has been evaluated in a Phase I clinical study on healthy volunteers.
Clinical trials
In March 2009, Pharmasset has initiated Phase I clinical trials in the US to evaluate the safety and tolerability of the drug in treating Hepatitis C. The dosage of the drug used in the trials varied from 25mg to 800mg.
Preliminary results of the study have shown that there were no adverse events or discontinuations relating to the usage of the drug. Also, no clinically significant changes were reported in vital signs or ECGs.
The primary objective of the study was to assess the safety and pharmacokinetics of the drug in treating HCV-infected patients. The secondary objective was to evaluate the antiviral activity of the drug by measuring the change in HCV RNA. Three dose cohorts of the drug were evaluated during the study.
In January 2010, Pharmasset initiated a 28-day Phase IIa study on PSI-7977.
The study enrolled 63 chronic HCV infected patients. It focused on evaluating different doses of PSI-7977 in combination with Pegasys (peginterferon alfa 2a) and Copegus (ribavirin).
The primary efficacy endpoint of the trial was to find the proportion of patients achieving rapid virologic response (RVR) within 28 days of treatment.
Phase II preliminary safety and efficacy results of the drug were announced in May 2010. The study found that the HCV reached undetectable levels in 93% of the patients. The drug administered with Pegasys and Copegus once daily, proved to be safe and well tolerated.
Pharmasset initiated Phase IIb clinical trials in the US in August 2010, with 125 HCV-infected patients not treated previously. The primary endpoint of the study was to assess the safety and tolerability of PSI-7977 in combination with the current standard of care (SOC).
The 12-week trial will assess PSI-7977 200mg QD and 400mg QD in combination with pegylated interferon alfa 2a and ribavirin, the SOC in HCV patients. The final results of the study are expected to come by the first half of 2011.