Qtern (dapagliflozin and saxagliptin) is a combination drug with sodium/glucose cotransporter (SGLT) 2 and dipeptidyl peptidase 4 (DPP-4) inhibitors indicated for the treatment of type 2 diabetes.
Developed by AstraZeneca, Qtern received US Food and Drug Administration (FDA) approval in February this year as a supplementary to improved diet and exercise for better glycaemic control. The company also received marketing authorisation approval (MAA) for Qtern in July 2016 from the European Commission (EC) in Europe and EU member countries including Iceland, Liechtenstein, and Norway.
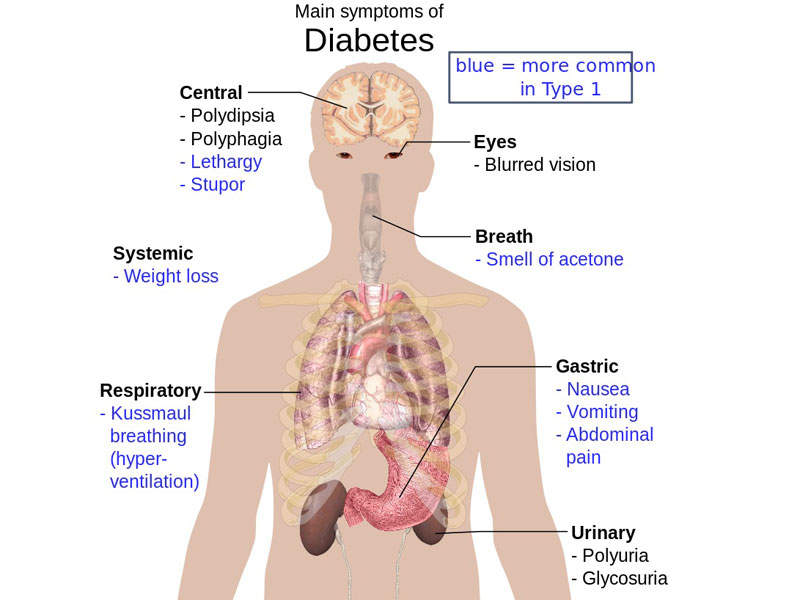
Type 2 diabetes causes and symptoms
Type 2 diabetes is a metabolic disorder characterised by high blood sugar levels in the body. The disease occurs when body cells do not use insulin efficiently, leading to an increase in blood sugar levels.
This condition may result in major problems such as heart disease, strokes, kidney failure, and diabetic retinopathy in the long-term.
Symptoms associated with type 2 diabetes include increased thirst and hunger, frequent urination, fatigue, sores, and unusual weight loss. It accounts for approximately 90% of all diabetes cases worldwide, with an estimated 29 million people in the US and more than 382 million people worldwide.
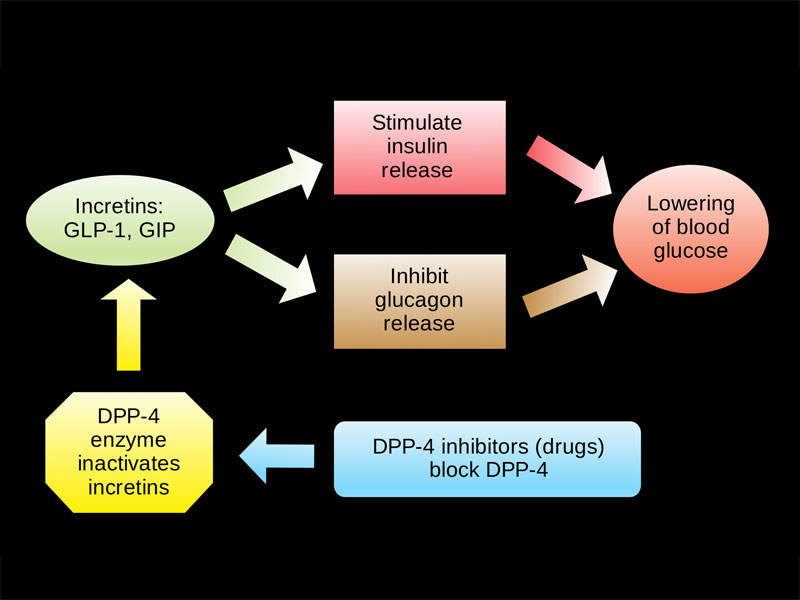
Qtern’s mechanism of action
Qtern contains anti-hyperglycaemic agents dapagliflozin and saxagliptin, which are respectively composed of sodium-glucose cotransporter 2 (SGLT-2) and dipeptidyl peptidase-4 (DPP-4) inhibitors.
The SGLT-2 inhibitor helps improve glycaemic control, reabsorbing filtered glucose and lowering the renal threshold.
The DPP-4 inhibitor slows inactivation of incretin hormones, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). This both increases bloodstream concentrations and reduces fasting and post-prandial glucose concentrations.
The drug is available in tablet form for oral administration.
Clinical trials on Qtern
The FDA and EC approvals for Qtern were based on data from a 24-week phase III clinical trial. This multi-centre, randomised, double-blind, active-controlled, parallel-group study was designed to evaluate the efficacy and safety of the combination of saxagliptin/dapagliflozin as dual add-on therapy. It enrolled 534 adult type-2 diabetics, with a baseline of HbA1c ≥ 8% and ≤ 12%.
Patients were randomised to receive either a combination of saxagliptin 5mg and dapagliflozin 10mg added to metformin, saxagliptin and metformin added to placebo, or dapagliflozin and metformin added to placebo.
The primary endpoint was a mean change in HbA1c, whereas secondary endpoints included a mean change from baseline in two-hour postprandial glucose (PPG) during a liquid meal test, fasting plasma glucose (FPG), and improved body weight at week 24.
The results from the combination study revealed patients treated with saxagliptin/dapagliflozin plus metformin achieved significantly greater reductions in HbA1c versus either agent alone plus metformin after 24 weeks.
Adjusted mean change from baseline in the saxagliptin/dapagliflozin combination group was -1.47%, compared to -0.88% in the saxagliptin group and -1.20% in the dapagliflozin group. The study also showed patients in the saxagliptin/dapagliflozin combination group achieved 41% goal HbA1c levels of less than 7%, compared to 18% patients in saxagliptin and 22% patients in dapagliflozin groups.
Study results further demonstrated that the adjusted mean reduction from baseline in two-hour PPG in the saxagliptin/dapagliflozin combination group was greater than that in the saxagliptin group. The adjusted mean reduction in FPG was -38mg/dL in the saxagliptin/dapagliflozin combination group, whereas it was -14mg/dL in the saxagliptin group and -32mg/dL in the dapagliflozin group.
Adverse events reported by all three groups during the study were mild or moderate in intensity.
In addition, results from two phase III clinical trials showed patients administered with a combination of saxagliptin and dapagliflozin added with metformin resulted in statistically significant reductions in HbA1c compared to patients treated with placebo.
Results from the third phase III study demonstrated that patients treated with combination of saxagliptin and dapagliflozin along with metformin resulted in statistically greater reductions in HbA1c compared to the patients treated with saxagliptin or dapagliflozin alone added to metformin.