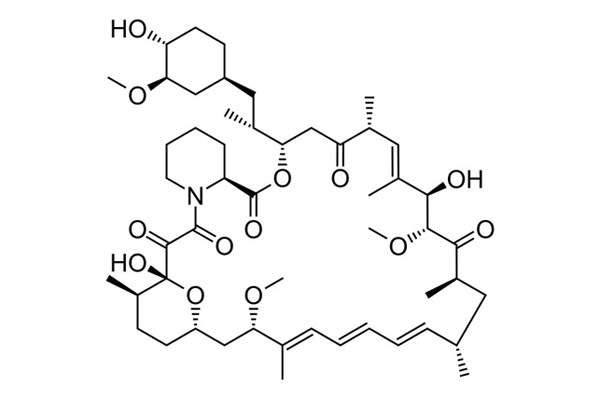
Rapamune (sirolimus), an immunosuppressive agent, is the first approved medicine in the US for the treatment of lymphangioleiomyomatosis (LAM), a rare, progressive lung disease that affects the lungs, kidneys and the lymphatic system.
The drug was discovered and developed by Pfizer.
The supplemental new drug application (sNDA) of Rapamune submitted by Pfizer to the US Food and Drug Administration (FDA) was approved under a priority review in May 2015. The approval made Rapamune the first treatment to stabilise lung function in lymphangioleiomyomatosis (LAM) patients.
The drug is also approved in the US for the prophylaxis of organ rejection in kidney transplant patients over the age of 13.
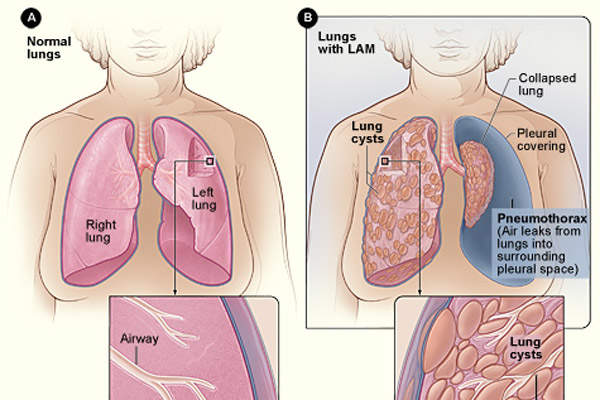
Lymphangioleiomyomatosis (LAM)
LAM is a rare, progressive lung disease characterised by an abnormal growth of smooth muscle cells in lung tissues that results in invading the airways, blood and lymph vessels. It leads to the obstruction of airflow and limits delivery of oxygen to the body over time.
LAM’s symptoms are similar to those caused by other lung diseases such as asthma, emphysema and bronchitis, making it difficult to diagnose. It is estimated that approximately 800 patients in the US are affected with LAM.
Rapamune’s mechanism of action
The active ingredient sirolimus inhibits the activation and proliferation of T-lymphocyte by a mechanism that is distinct from that of other immune-suppressants. It also inhibits antibody production and binds to immunophilin to generate an immunosuppressive effect.
Rapamune is available in two forms, an oral solution and tablets. Each oral solution carton contains a 60ml solution in an amber glass bottle, one oral syringe adapter, disposable amber oral syringes with caps and a carrying case. The solution has to be mixed with water or orange juice for oral administration only.
Rapamune tablets are available in yellow-to-beige colour in 0.5mg, 1mg and 2mg dosages.
Clinical trials on Rapamune
Zykadia (Ceritinib / LDK378) is an inhibitor of anaplastic lymphoma kinase (ALK) indicated for treatment of non-small cell lung cancer (NSCLC).
FDA approval was based on results from the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus trial (MILES).
Results of the study showed patients treated with Rapamune for one year experienced stabilisation of lung function when measured by forced expiratory volume in one second (FEV1).
Pfizer conducted a randomised, double-blind, multi-centre, controlled study to determine Rapamune’s safety and efficacy in treating LAM.
The study, which enrolled 89 LAM patients with moderate lung impairment, compared Rapamune with a placebo for a one year treatment period followed by one year observation period. Out of 89 enrolled patients, 43 received a placebo and 46 received Rapamune.
The primary endpoint was the difference between the rate of change (slope) per month in FEV1 in the two groups.
During the treatment period, the difference between the groups was 13ml, while the FEV1 slope was -12±2ml a month in the placebo group and 1±2ml a month in the Rapamune group.
The mean change in FEV1 during the one year period was 153ml. It was observed that decline in lung function was resumed after discontinuing Rapamune.
In addition, the rate of changeover of vascular endothelial growth factor D (VEGF-D), which is a lymphangiogenic growth factor shown to be elevated in patients with LAM, was significantly different in the Rapamune-treated group compared to the placebo group.
The mean change in VEGF-D during the one-year period was approximately 50% of the mean VEGF-D at enrolment.
The most common adverse reactions observed during the trial were stomatitis, diarrhoea, abdominal pain, nausea, nasopharyngitis, acne, chest pain, peripheral oedema, upper respiratory tract infection, headache, dizziness, myalgia and hypercholesterolemia.