REGEN-COV™ (casirivimab with imdevimab) is an investigational monoclonal antibody cocktail authorised for the treatment of mild to moderate Covid-19 in adults and paediatric patients aged 12 years and older who are infected with SARS-CoV-2 and are at high risk of progression to severe Covid-19.
REGEN-COV has been developed by Regeneron Pharmaceuticals, partially funded by the US Department of Health and Human Services’ Biomedical Advanced Research and Development Authority (BARDA) for development and manufacturing.
Regeneron partnered with Roche in August 2020 to increase the global supply of the therapy. Regeneron is responsible for distributing REGEN-COV in the US, while Roche will distribute the therapy outside the US. The companies plan to manufacture at least two million treatment doses a year once they reach full manufacturing capacity in 2021.
Casirivimab and imdevimab are available in single-dose vials as a 300mg/2.5mL (120mg/mL) or 1332mg/11mL (120mg/mL) sterile, preservative-free aqueous solution to be diluted prior to intravenous infusion.
REGEN-COV approvals
In November 2020, the US Food and Drug Administration (FDA) authorised casirivimab and imdevimab for emergency use for the treatment of mild to moderate Covid-19 patients.
In May 2021, Roche India received an emergency use authorisation (EUA) for the antibody cocktail from India’s Central Drugs Standards Control Organisation (CDSCO) to treat mild to moderate Covid-19 in high-risk patients. The antibody cocktail will be distributed across India through a strategic partnership with Cipla, a leading Indian pharmaceutical company.
The CDSCO’s authorisation was based on the data submitted for the EUA in the US, as well as the scientific opinion of the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP).
Covid-19 causes and symptoms
Covid-19 is a respiratory infection caused by SARS-CoV-2, a coronavirus that emerged in December 2019. The virus has caused millions of deaths worldwide, as well as long-term health complications among those who have survived the disease.
Covid-19 is contagious and transmits from person to person. The symptoms appear in patients two to 14 days after being exposed to the infection. An individual infected with Covid-19 becomes infectious for others up to two days before the occurrence of any symptoms, and remains contagious for ten to 20 days afterwards depending on the severity of their infection and their immune system.
Covid-19 symptoms include cough, fever, difficulty breathing, body aches, sore throat, loss of taste or smell, diarrhoea, headache, fatigue, vomiting and congestion.
Some individuals infected with Covid-19 have a mild infection, while others experience no symptoms at all. Covid-19 can also cause respiratory failure, long-term lung and heart muscle damage, nervous system complications, kidney failure and, in some cases, death.
REGEN-COV’s (casirivimab with imdevimab) mechanism of action
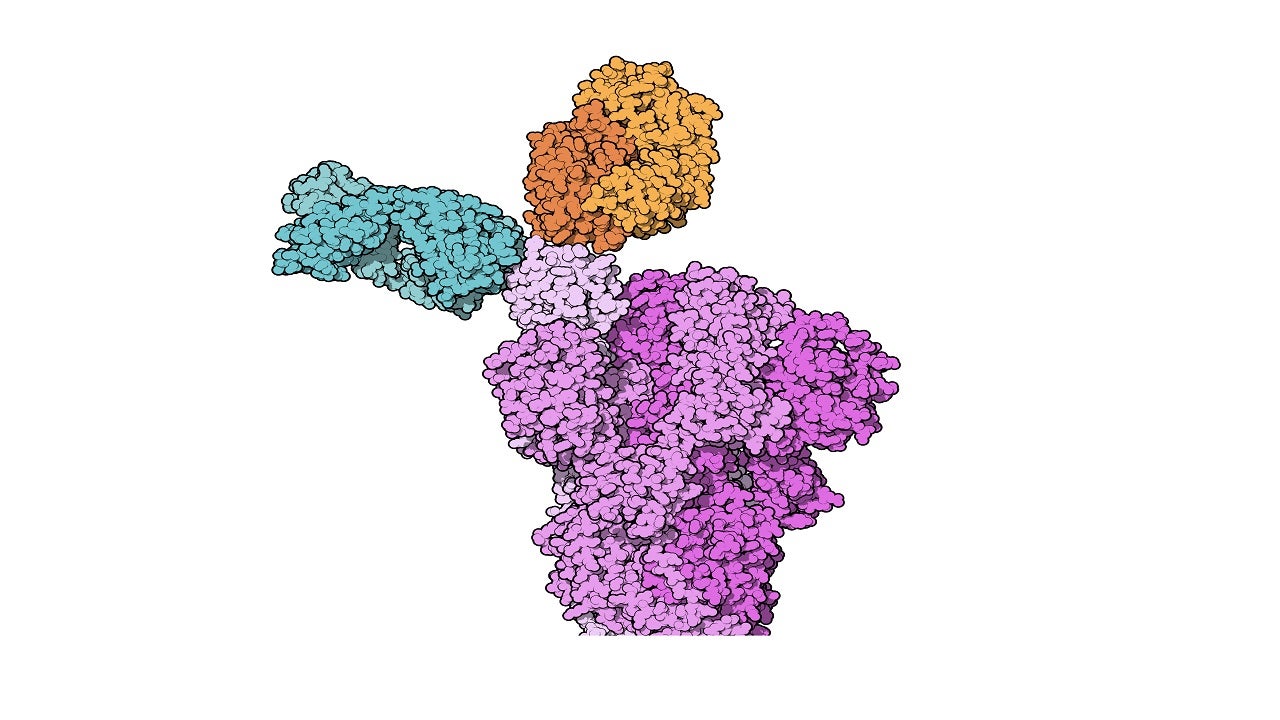
Casirivimab and imdevimab are human immunoglobulin G-1 (IgG1) monoclonal antibodies developed using recombinant DNA technology.
The monoclonal antibodies are explicitly directed against the spike protein of SARS-CoV-2, preventing the virus from attaching to and entering human cells.
The specific engineering of the two neutralising antibodies allows them to bind to different parts of the virus spike. This enables them to remain effective against various existing strains and decrease the risk of losing neutralisation potency against new emerging variants.
Clinical trials on REGEN-COV
The EUA for casirivimab and imdevimab was based on the results of a randomised, double-blind, placebo-controlled clinical trial. In the trial, 799 non-hospitalised adults with mild to moderate Covid-19 symptoms were enrolled to evaluate the therapy’s safety profile.
Out of the 799 patients, 266 patients received 2,400mg of casirivimab and imdevimab (1,200mg of each), 267 patients received 8,000mg of casirivimab and imdevimab (4,000mg of each), and 266 patients received a placebo. Each of these was given within three days of a positive Covid-19 test.
The trial’s primary endpoint was the viral load reduction in patients.
At day seven, the drop in viral load in patients treated with casirivimab and imdevimab was greater than that in those patients treated with placebo.
The predefined secondary endpoint of medically attended visits related to Covid-19, especially hospitalisations and emergency room (ER) visits within 28 days after treatment, provided the strongest evidence for the efficiency of the co-administration of casirivimab and imdevimab.
Hospitalisations and ER visits were observed in 3% of casirivimab and imdevimab-treated patients on average, compared to 9% of placebo-treated patients at high risk for disease progression.
Patients receiving either of the two casirivimab and imdevimab doses had similar effects on viral load, hospitalisations and ER visits.
The serious adverse events reported in patients during the trial were pneumonia, hyperglycaemia, nausea and vomiting, intestinal obstruction and dyspnoea, pneumonia, and hypoxia.