Rifapentine is an antibiotic drug developed by Sanofi-aventis for treating tuberculosis (TB). It is marketed under the brand name Priftin and was approved by the US Food and Drug Administration (FDA) in June 1998.
In October 2008, Sanofi-aventis and the Global Alliance for TB Drug Development (TB Alliance) entered into an agreement to accelerate the discovery, development and clinical use of drugs against tuberculosis. Rifapentine was granted orphan drug status for the treatment by the European Commission on 1 July 2010.
Tuberculosis
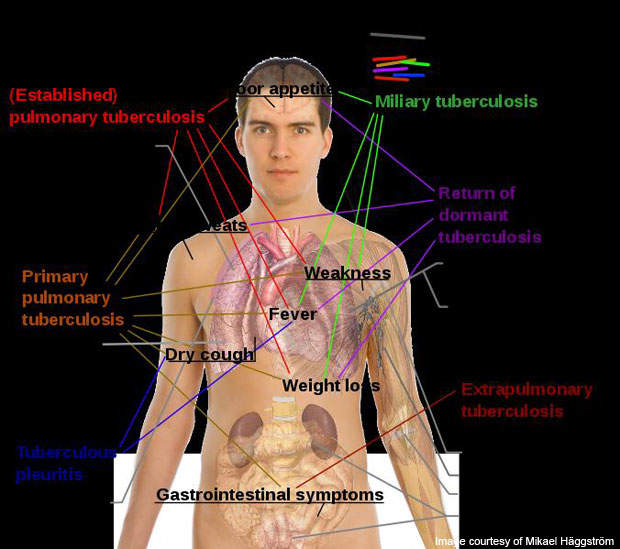
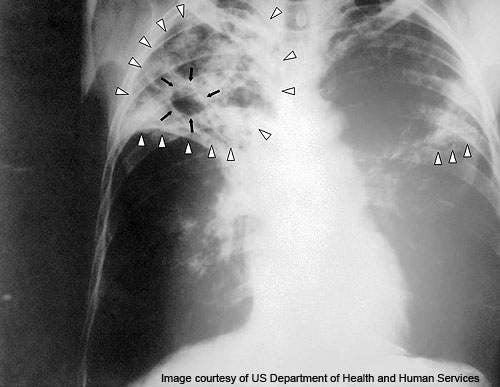
TB is a life-threatening contagious disease that is caused by mycobacterium tuberculosis bacterium. TB bacteria can attack the lungs and other organs such as the spine, brain and kidneys. The disease can be spread from one person to another through the air. The most common symptoms of the disease are coughing up phlegm or blood, excessive sweating during nights, fatigue and sudden weight loss.
In 2007 an estimated 13.7 million were affected by chronic TB. Since then, 1.8 million have died. In 2008 the World Health Organisation (WHO) estimated that 34% of cases were registered in South East Asia.
The estimated incidence of TB cases in sub-Saharan Africa, however, is more than that of South-East Asia with more than 350 cases per 100,000 people. In the US, incidents of TB were reported to be about ten cases per 100,000 people. The prevalence of the disease is highest in India, with more than 1.3 million new cases every year.
Using rifapentine to target TB
Rifapentine can be administered orally and contains the active ingredient rifapentine. The drug also contains calcium stearate, disodium EDTA, microcrystalline cellulose, hydroxypropyl cellulose and sodium ascorbate.
The drug can be used along with daily companion drugs within a six-month treatment. The dose of the drug can be twice-a-week during the intensive phase and once-a-week in the continuation phase
Clinical trials
Phase I clinical trials began in April 2010 and are expected to be completed by May 2011. The study enrolled 36 TB patients with the objective of evaluating the safety and tolerability of the drug. The drug will be administered in escalating doses to observe pharmacokinetics.
In the primary outcome measure, the safety of the drug will be determined by orally administering 5-20mg/kg doses to healthy volunteers for 14 days. In the secondary outcome measure, the effect of increasing doses of RPT on autoinduction of RPT metabolism will be observed.
In April 2008 Sanofi-aventis enrolled 480 TB patients from 29 locations in the US for phase II clinical trials. The primary objective of the study is to evaluate antimicrobial activity and safety of an experimental tuberculosis treatment regimen in which the drug is substituted for rifampin. The secondary objective of the study is to compare and determine each regimen proportion of patients with Grade 3 or 4 adverse reactions.