RYSTIGGO® (rozanolixizumab-noli) is a targeted therapy indicated for adults with generalised myasthenia gravis (gMG) who are anti-acetylcholine receptor or anti-muscle-specific tyrosine kinase antibody positive.
Developed by UCB, a biopharmaceutical company based in Belgium, RYSTIGGO is available in a single-dose vial containing 280mg/2ml (140mg/ml) of colourless to light brownish yellow solution for subcutaneous injection.
The drug was made commercially available in the US in July 2023. UCB offers ONWARD™, a personalised patient support programme, to eligible patients and caregivers, providing assistance throughout the treatment with UCB’s rare disease medications, including RYSTIGGO.
Regulatory approval for RYSTIGGO
The drug received approval in Japan for the treatment of gMG in adult patients, who inadequately respond to steroids or other immunosuppressants, in September 2023. It also holds an orphan drug designation in Japan.
In June 2023, the US Food and Drug Administration granted approval to the drug for the treatment of gMG, following the orphan drug designation granted in 2019.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended RYSTIGGO’s marketing authorisation approval for the treatment of gMG in November 2023.
RYSTIGGO was granted orphan drug designation by the European Commission in April 2020.
Generalised myasthenia gravis causes and symptoms
gMG is a rare, chronic, and unpredictable autoimmune disease characterised by malfunction and damage at the neuromuscular junction (NMJ). The prevalence of gMG is 100 to 350 cases per million globally. gMG may affect anyone of any age, gender or colour.
In gMG, detrimental autoantibodies may hinder synaptic transmission at the NMJ by focusing on particular proteins located on the post-synaptic membrane. It interferes with the nerve’s capacity to activate the skeletal muscle and results in weakened muscular contraction.
The symptoms include double vision, drooping eyelids, extreme muscle weakness, trouble speaking, eating and swallowing and life-threatening weakness of the respiratory muscles.
RYSTIGGO’s mechanism of action
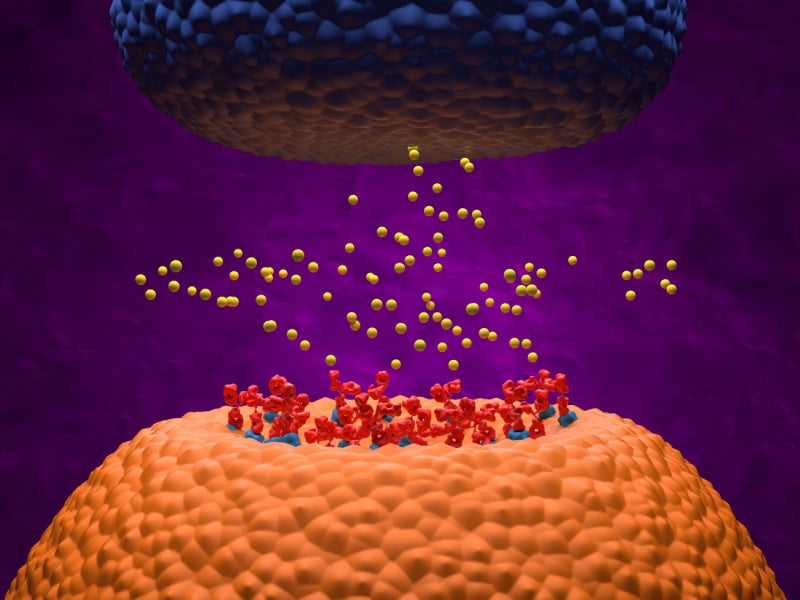
RYSTIGGO is a humanised IgG4 monoclonal antibody that binds to the human neonatal Fc receptor (FcRn) and blocks the interaction between FcRn and Immunoglobulin G (IgG).
The process increases the rate at which antibodies are catabolised and decreases the concentration of pathogenic IgG autoantibodies. It promotes intracellular IgG degradation, resulting in the reduction of circulating IgG.
Clinical trials on RYSTIGGO
The approval of the drug in the US and Japan was based on safety and efficacy results from the pivotal Phase Ⅲ MycarinG clinical trial.
This was a multi-centre, randomised, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of the drug in adult patients with gMG.
The primary efficacy endpoint of the study was the comparison of the change from baseline between treatment groups in the Myasthenia Gravis-Activities Daily Living Profile (MG-ADL) total score at day 43.
The MG-ADL measures the effects of gMG on eight daily activities such as breathing, speaking, swallowing and being able to rise from a chair. Every item is evaluated using a four-point scale, where a rating of zero signifies normal functioning and a rating of three indicates a complete inability to perform the respective function. The overall score spans from zero to 24, with higher scores suggesting greater impairment.
The reductions in MG-ADL score from baseline to day 43 were more significant in the rozanolixizumab 7mg/kg group and the rozanolixizumab 10mg/kg group compared to the placebo group.
Secondary efficacy endpoints included the change from baseline to day 43 in the Quantitative Myasthenia Gravis (QMG), a 13-item categorical grading system that assesses muscle weakness.
A statistically significant difference was observed favouring rozanolixizumab-noli compared to placebo.
The most common adverse reactions reported in the trial were headaches, infections, diarrhoea, pyrexia, hypersensitivity reactions and nausea.