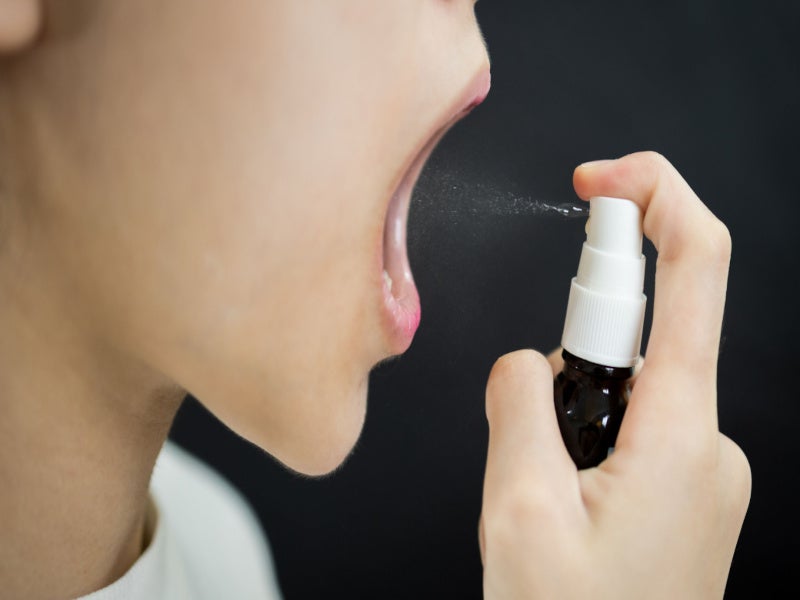
Sativex®, also known as Nabiximols, is an oromucosal spray comprising two chemical extracts – active cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC) – derived from the cannabis plant.
It is used for treating spasticity due to multiple sclerosis (MS), neuropathic pain and intractable cancer pain.
Sativex was developed by GW Pharmaceuticals, a UK-based pharmaceutical company. GW Pharmaceuticals was later acquired by Jazz Pharmaceuticals, a pharmaceutical company based in Ireland, in May 2021.
GW Pharmaceuticals teamed up with several companies to support the development and commercialisation of the drug in different parts of the world. It collaborated with Otsuka for development in the US, with Almirall in Europe (except the UK) and Mexico. It collaborated with Novartis for development in Australia, New Zealand, parts of Asia, the Middle East (except Israel), and Africa and with Bayer for development in the UK and Canada. Ipsen covers Latin America (except Mexico and the Caribbean islands) while Neopharm handles Israel.
Subsequently, GW Pharmaceuticals dropped its licence deal with Otsuka for Sativex in the US in December 2017, reclaiming control of the product’s research and marketing rights without upfront payments. It also reclaimed the exclusive commercialisation rights to Sativex in the UK from Bayer in March 2020.
Sativex is available in a 10ml vial containing up to 90 metered sprays. It is available as a spray solution for use as an oro-mucosal or buccal pump spray. The metering pump delivers 100 microlitres per spray. Each carton includes one, two, three, four, five, six, eight, ten, or 12 amber glass vials.
Regulatory approvals for Sativex
Sativex received regulatory approval for the treatment of neuropathic pain associated with MS in Canada in 2005. In August 2007, Canadian regulators approved Sativex as an adjunctive analgesic treatment in adult patients with advanced cancer pain.
Almirall submitted a regulatory application for Sativex for the treatment of MS spasticity in the UK and Spain in May 2009. The application was approved by the Medicines and Healthcare Products Regulatory Agency (MHRA) in June 2010. GW Pharmaceuticals received approval for Sativex for treating MS-related spasticity in Spain in July 2010.
Sativex was approved by New Zealand’s regulatory authority MedSafe in November 2010.
The company announced the successful completion of the European Mutual Recognition Procedure (MRP) for Sativex, leading to the drug’s approval in Italy, Denmark, Germany, Sweden, the Czech Republic and Austria, in March 2011.
The drug currently has approvals in 29 countries for treating adult patients experiencing moderate to severe spasticity caused by MS, particularly those who haven’t found sufficient relief with other anti-spasticity medications.
Multiple sclerosis causes and symptoms
MS is a chronic, inflammatory demyelinating disease affecting the central nervous system. Some of its common symptoms include vision problems, diplopia, dysarthria, ataxia, cognitive impairment, and sexual dysfunction.
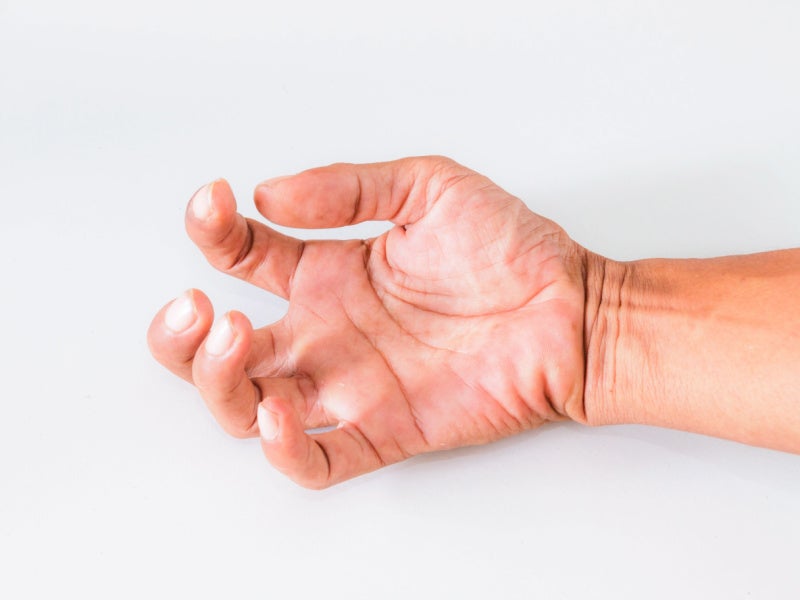
Patients may experience Lhermitte’s phenomenon, a brief electrical sensation in the back caused by neck flexion. Spasticity, including muscular spasms and stiffness, is a common symptom of MS, affecting daily activities such as walking and sitting. Other symptoms include limb weakness, tingling, and tight sensations.
Multiple sclerosis may result from autoimmune disorders, infectious agents, environmental factors, or genetic factors.
Sativex’s mechanism of action
CBD is an agonist of the TRPV-1 (vanilloid) receptor, inhibiting adenosine uptake. The human endocannabinoid system contains two types of cannabinoid receptors: CB₁ at nerve terminals in the central nervous system, peripheral tissues and immune cells.
CB₁ receptors are relevant for pain modulation, as they are found on pain pathways in the brain and spinal cord, as well as on peripheral nervous system primary afferent neurons.
CB₂ receptors are primarily present on peripheral and central immune cells, modulating immune function through cytokine release.
THC, the main psychotropic component of cannabis, acts as a partial agonist at both CB₁ and CB₂ receptors. In animal models of MS and spasticity, CB receptor agonists have been shown to improve limb stiffness and motor function.
CBD, which has little activity at cannabinoid receptors, has neuroprotective properties, likely due to its ability to modulate intracellular calcium. CBD’s key pharmacology in MS is likely to inhibit microglial activity and T-cell proliferation. It is unclear whether CBD in Sativex has a facilitative or antagonising effect on THC’s anti-spasticity action.
Clinical trials on Sativex
The pivotal Phase III clinical trial served as the basis for the approval of the drug in the UK and Spain.
The trial is a double-blind, randomised, placebo-controlled study of Sativex in individuals with MS-related spasticity who had not received a sufficient reduction of their symptoms from other treatments.
The trial’s primary efficacy endpoint was achieved, demonstrating that Sativex provides statistically significant benefits. Various additional measurements, including the analysis of responders experiencing at least a 30% improvement; reduction in spasm frequency; decrease in sleep disruption; overall impressions of improvement reported by both patients and physicians; and the disparity between Sativex and a placebo; also showed considerable significance of the benefits.
The most common adverse side effects were temporary drowsiness or vertigo, generally of a mild to moderate degree.