Steglatro™ (ertugliflozin) is a combination drug indicated for the improvement of glycaemic control in adult patients with Type 2 diabetes mellitus.
The drug was jointly developed by Merck and Pfizer under a collaboration formed in 2013.
The US Food and Drug Administration (FDA) approved Steglatro™ in December 2017. The marketing authorisation application (MAA) for the drug was accepted for review by the European Medicines Agency (EMA) in December 2017.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) gave a positive opinion and recommended for the drug’s approval in January 2018.
Type 2 diabetes causes and symptoms
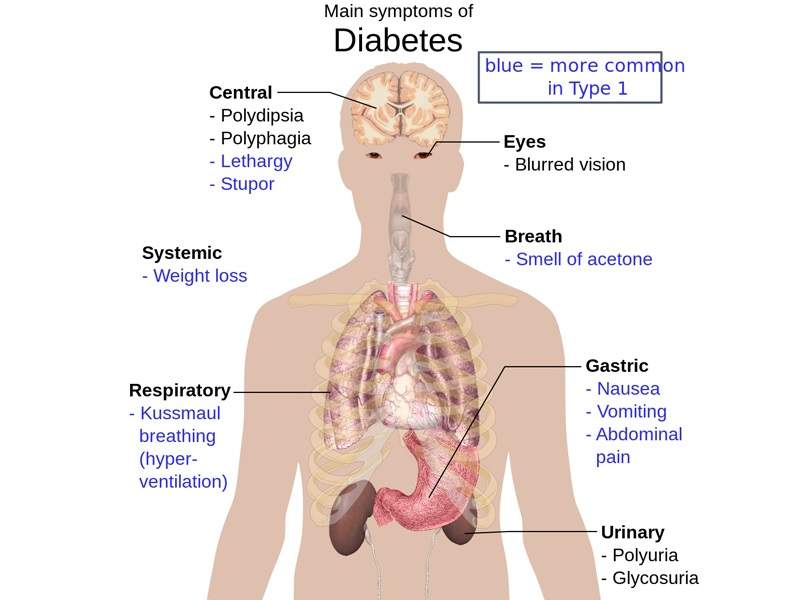
Type 2 diabetes is a metabolic disorder resulting from high blood glucose or blood sugar levels that occur when body cells do not use insulin efficiently. It can lead to the onset of cardiovascular disease.
The most common symptoms associated with Type 2 diabetes are increased thirst, frequent urination, unusual weight loss, fatigue and increased hunger. The symptoms are found in more than 85% to 95% of adult patients diagnosed with diabetes.
The disease may also lead to long-term problems such as heart disease, strokes, diabetic retinopathy and kidney failure.
Diabetes is estimated to affect 30 million people in the US. Of these, one third have Type 2 diabetes.
Steglatro’s mechanism of action
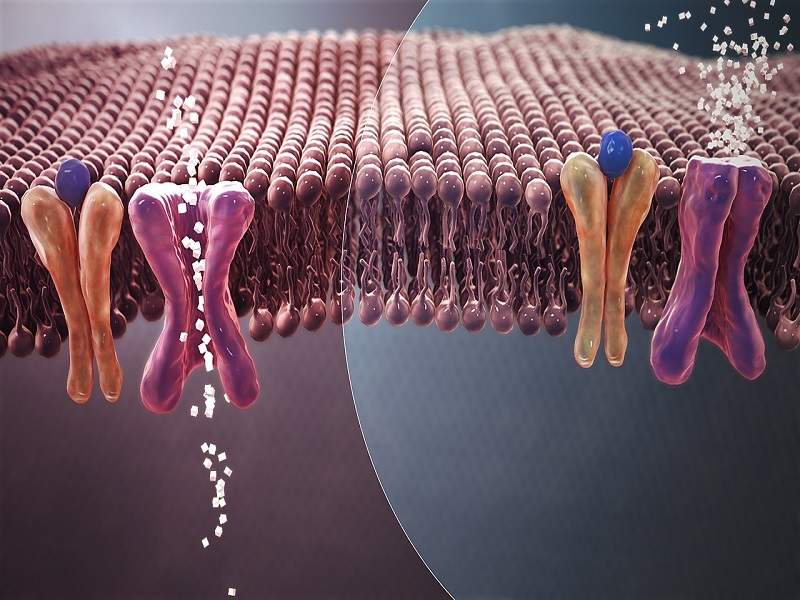
Steglatro™ contains ertugliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor. SGLT2 is responsible for reabsorption of glucose from the glomerular filtrate. Ertugliflozin decreases renal reabsorption of filtered glucose by inhibiting SGLT2 and increases glucose excretion.
Steglatro™ is available in 5mg and 15mg tablets, which can be administered orally.
Clinical trials on Steglatro
The FDA’s approval of Steglatro™ was based on seven Phase III studies conducted on 4,863 patients with Type 2 diabetes mellitus. The average age of the enroled patients was 57.8 years.
The trials evaluated Steglatro™ both as a monotherapy and in combination with metformin, and in combination with a dipeptidyl peptidase 4 (DPP-4) inhibitor in adults with Type 2 diabetes and moderate renal impairment.
The drug was also studied in combination with insulin and sulfonylurea. The primary endpoint of the studies was statistically significant reductions in hemoglobin A1c (HbA1c).
The Steglatro™ monotherapy study enrolled 461 patients with Type 2 diabetes, while the clinical study in combination with metformin enrolled 621 patients. At the 26th week, patients taking Steglatro™ reached the primary endpoint with significant reductions in HbA1c compared to placebo.
Steglatro™ (5mg/15mg) in combination with Sitagliptin and Sitagliptin alone was studied in 1,233 patients. Steglatro™ (5mg/15mg) in combination with Sitagliptin 100mg resulted in significant reduction in HbA1c compared to Steglatro™ (5mg/15mg) alone or Sitagliptin (100mg) alone.
One of the major studies supporting the FDA approval of Steglatro™ was a double-blind, placebo-controlled study named VERTIS SITA2, which compared Steglatro™ to placebo in 463 patients with Type 2 diabetes on background metformin (≥1,500 mg/day) and sitagliptin (100mg/day) therapy.
The trial randomised patients to take Steglatro™ 5mg/15mg or placebo administered once daily.
VERTIS SITA clinical trials met the primary endpoint of a statistically significant decrease in HbA1c when used alone or administered with sitagliptin. The clinical trials also showed a significant decrease in body weight, fasting plasma glucose (FPG), diastolic blood pressure (DBP) and systolic blood pressure (SBP).
The most common adverse reactions found in more than 5% of patients during the clinical studies were female genital mycotic infections.