Sutent (sunitinib malate) is an oral multikinase inhibitor which is indicated for the treatment of progressive, well-differentiated pancreatic neuroendocrine tumours (NET). It is manufactured by Pfizer.
Pfizer submitted the supplemental new drug application (NDA) of sutent for treating pancreatic NET to the US Food and Drug Administration (FDA) in December 2009. Pfizer received a Complete Response letter from the FDA in May 2010.
In May 2011, sutent was approved by the US FDA (Food and Drug Administration) for the treatment of pancreatic NET in patients with unresectable locally advanced or metastatic disease. The drug was also approved in Europe and nine other countries, for the treatment of advanced pancreatic NET.
Rarity and rates of pancreatic neuroendocrine tumours (NET)
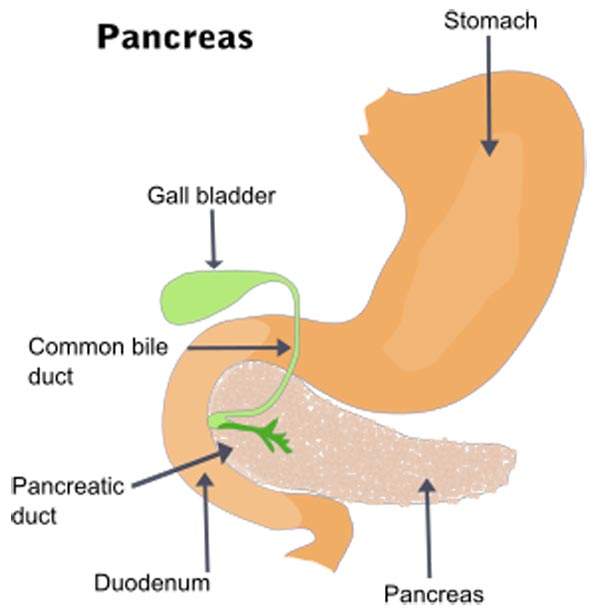
Pancreatic neuroendocrine tumours (NET), also known as islet cell tumours, are a group of rare tumours of the endocrine pancreas. They form due to the hormone-producing cells in the pancreas. About 90% of the non-functional neuroendocrine tumours are cancerous.
It was found that about 90% of the patients with pancreatic NET were initially diagnosed with locally advanced or metastatic cancer. Patients with pancreatic NET in advanced or metastatic stage can survive for one to three years.
The disease is a rare cancer reported in two to four people per million each year worldwide.
Multiple molecular growth receptors blocked by sutent
The drug contains an oral multikinase inhibitor which blocks multiple molecular growth receptors and stops the spread of cancer. The drug stops growth of vascular endothelial growth factor receptors (VEGFR), platelet-derived growth factor receptors (PDGFR) and KIT.
Clinical trials carried out on Pfizer’s oral multikinase inhibitor
Pfizer has conducted clinical trials on sutent in more than 100 countries and in excess of 100,000 patients were treated with the drug worldwide.
Phase I / II clinical trials were conducted between January 2005 and August 2008. The open label, non-randomised and single group study enrolled 36 patients in Japan. The primary outcome measure of the study was to find the number of patients with dose limiting toxicities, while the secondary outcome measures included finding concentrations of vascular endothelial growth factor (VEGF).
Phase III clinical trials on sutent were conducted between June 2007 and April 2009. The FDA approval for sutent was based on a Phase III clinical trial known as the SUN 1111 study. It was a multicentre, randomised, double-blind and placebo-controlled clinical study. It enrolled 171 patients with PNET. The patients were randomised with sutent 37.5mg and placebo once daily. The primary endpoint of the study was progression-free survival (PFS).
The results of the study showed that the median PFS in sutent-administered patients was 10.2 months, while in placebo-administered patients it was 5.4 months. The objective response rate in sutent arm was 9.3%, where as in placebo arm it was 0%. In the sutent arm nine deaths were reported, while in placebo group 21 patients died.
Related project
Dulera – Treatment for Asthma, United States of America
Dulera (mometasone furoate and formoterol fumarate dihydrate) is a new fixed-dose combination inhaler for the treatment of asthma.
About 22% of the patients treated with sutent discontinued the clinical study due to adverse events, whereas in the placebo group it was 17%. The common adverse reactions found in the clinical studies on sutent included anorexia, asthenia, hypertension, nausea, diarrhoea, vomiting, changes in taste and hair colour, bleeding, skin discoloration and fatigue.
In September 2010, Pfizer discontinued a Phase III clinical trial named SUN 1120, which had been intended to evaluate sutent in combination with prednisone for men with advanced castration-resistant prostate cancer (CRPC). The study was discontinued as the interim data showed that the combination of sunitinib with prednisone was unlikely to improve overall survival chances.
Marketing commentary on sutent (sunitinib malate) and competitors
Sutent is the first anti-VEGF therapy approved by the FDA for the treatment of pancreatic NET. In 2006, Pfizer received the approval for sutent from the FDA for treating advanced kidney cancer (RCC) and intolerant gastrointestinal stromal tumours (GIST).
Other drugs approved by the FDA for the treatment of PNET include Zanosar (streptozocin), manufactured by Teva Pharmaceuticals in 1982, and Sandostatin (octreotide acetate), produced by Novartis in 1998.
Related content
Erwinaze (Asparaginase Erwinia Chrysanthemi) – Treatment for ALL, United States of America
Erwinaze (asparaginase Erwinia chrysanthemi) is indicated for the treatment of acute lymphoblastic leukaemia (ALL). It is manufactured by EUSA Pharma.
Small players, big drugs – pharmaceutical SMEs take the innovative edge
Medical advances are leading to more novel therapeutics but it’s small biotech, not Big Pharma, which stands to gain the most.