AN2728 is an anti-inflammatory drug being developed by Anacor Pharmaceuticals as a treatment for psoriasis.
Anacor is currently conducting a Phase IIb clinical trial to evaluate the drug’s safety and efficacy in patients with mild to moderate psoriasis. The results of this study will determine the design and size of two proposed pivotal Phase III trials, which are expected to be launched in the second half of 2011.
If approved, AN2728 will be the first topical, non-steroidal treatment for the condition.
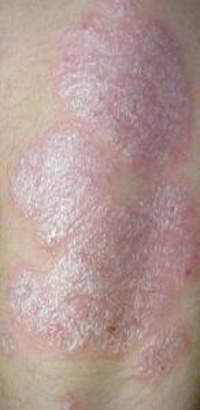
Psoriasis
Psoriasis is a chronic skin disorder that affects elbows, knees, scalp and genital area. The disease is caused by new skin cells that are formed due to faulty signals in the immune system, and is graded as mild, moderate or severe based on its effect on the body. The disease can also cause inflammations of the joints, which is known as psoriatic arthritis.
The condition affects more than 7.5 million people in the US and more than 100 million worldwide.
Targeting psoriasis
AN2728 includes a boron-based small-molecule compound, which works by suppressing the bond-breaking activity of an enzyme called phosphodiesterase-4. In turn, the suppression reduces the production of tumour necrosis factor-alpha, a precursor to inflammation associated with psoriasis. The drug also slows down excessive cell reproduction.
Clinical trials
Anacor has conducted three Phase 1b clinical trials on AN2728. The first, conducted in Germany in November and December 2007, was a randomised, observer-blind study involving 12 male patients with psoriasis. The purpose of the study was to test the efficacy of different concentrations of AN2728; the drug met the study’s primary endpoint, and the efficacy and safety of AN2728 were also tested.
The second Phase Ib clinical trial was also a randomised and observer-blind study involving 12 patients, and was conducted in February and March 2008. The purpose of the study was to investigate the dose-response relationship of AN2728. The study showed a significant activity over vehicle.
The third Phase Ib clinical trial was a randomised, double-blind and vehicle-controlled study. It was conducted between November and December 2010 on 16 patients in Australia. The purpose of the study was to assess the quantity of drug absorbed by the body after being applied to the skin. The study results showed that 2% AN2728 topical ointment was well-tolerated.
Phase IIa clinical trials were conducted between November 2007 and March 2008. The randomised, double-blind and bilateral study enrolled 35 patients. The results proved the efficacy and safety of the drug, which met the study’s primary and secondary endpoints. More than 69% of the patients treated with AN2728 scored better at the end of the therapy.
Anacor initiated double-blind, randomised and bilateral Phase II clinical trials on AN2728 between September and December 2008. The study enrolled 30 patients. AN2728 achieved a lower overall target plaque severity score in a significantly greater proportion of patients.
Anacor started Phase IIb clinical trial to evaluate the safety and efficacy of AN2728 in patients with mild to moderate psoriasis on AN2728 in February 2011. The multi-centre, double-blind study enrolled 68 patients randomised in a 2:1 ratio, AN2728 to vehicle.
In June 2011, Anacor announced positive preliminary results from the study. The results demonstrated that patients treated with AN2728 achieved 26% efficacy compared to an efficacy of 18% in the vehicle arm.
Marketing Commentary
According to LeadDiscovery, psoriasis drug sales in the US, Japan, Germany, France, UK and Spain were $42.5bn in 2008. It is estimated that in 2009 more than 3.9 million patients were prescribed topical therapies in the US.