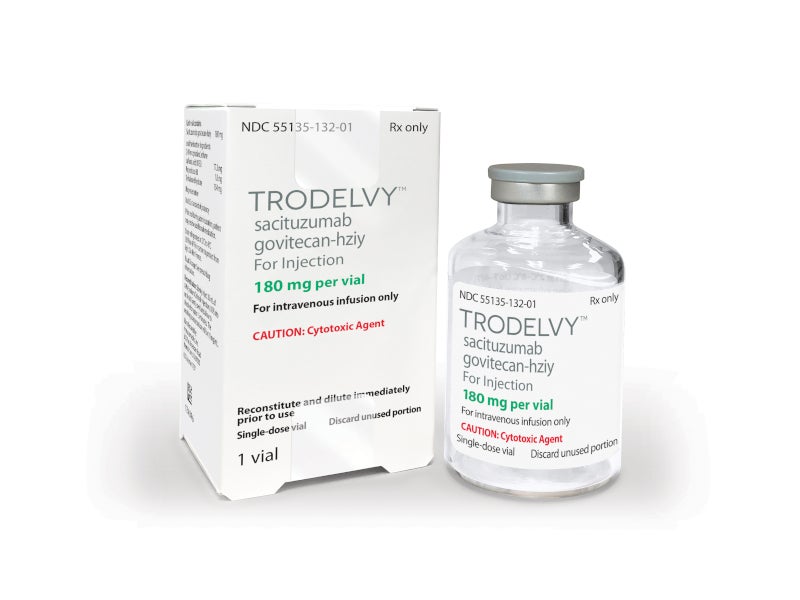
Trodelvy (sacituzumab govitecan-hziy) is the first approved antibody-drug conjugate for the treatment of advanced triple-negative breast cancer (TNBC) in adult patients.
Patients who received at least two therapies for the metastatic disease earlier are eligible for the treatment.
Currently, Trodelvy is indicated for both pre-treated HR+/HER2- metastatic breast cancer and second-line metastatic TNBC patients who have received endocrine-based therapy and at least two additional systemic therapies.
The drug is also used to treat locally advanced or metastatic urothelial cancer (mUC) patients who have previously received a platinum-containing chemotherapy and either a programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PDL1) inhibitor.
The drug was developed by Immunomedics, a biotech company, which was acquired by US-based biopharmaceutical company Gilead Sciences for $21bn in October 2020.
Trodelvy is available for intravenous administration as 180mg off-white to yellow-coloured lyophilised powder in single-dose vials for reconstitution.
Regulatory approvals for Trodelvy
Immunomedics submitted a biologics licence application (BLA) for the drug to the US Food and Drug Administration (FDA) in May 2018. The application was rejected due to several developmental and chemical concerns. The company resubmitted the BLA after addressing the concerns for accelerated approval in December 2019.
Immunomedics collaborated with Everest Medicines for the development, registration and commercialisation of sacituzumab govitecan in Greater China, South Korea and some Southeast Asian territories in April 2019.
Trodelvy received fast track designation for the treatment of advanced or locally metastatic urothelial cancer in April 2020. It received accelerated approval from the FDA to treat advanced TNBC in adult patients in the same month. The drug also holds breakthrough therapy status.
The FDA approved Trodelvy as the first treatment for metastatic TNBC in April 2021.
In November 2021, Trodelvy was approved in Europe for the treatment of metastatic TNBC in patients who have received two or more prior systemic therapies.
In August 2022, Gilead Sciences acquired the remaining global rights from Everest Medicines for the development and commercialisation of Trodelvy in countries, such as China, South Korea, Singapore, Indonesia, Philippines, Vietnam, Thailand, Malaysia, and Mongolia.
In February 2023, Trodelvy received FDA approval for pre-treated HR+/HER2 metastatic breast cancer in adult patients who had received endocrine-based therapy and at least two additional systemic therapies.
Trodelvy was approved for the same indication in Europe in July 2023. The drug is now approved in more than 40 countries.
Triple-negative breast cancer causes and symptoms
TNBC is an aggressive form of cancer, which accounts for approximately 20% of all types of breast cancers.
Patients with TNBC do not have any receptors, typically oestrogen receptors, progesterone receptors and human epidermal growth factor receptor 2 (HER2), which are generally expressed in breast cancers. The lack of receptors makes the disease difficult to treat.
The disease affects both men and women equally and patients are prone to relapse within the first three years of treatment. People of African American or Latin origin, women under 40 and those with the BRCA gene mutation are more likely to be at risk of TNBC.
The most common symptoms of the disease are a lump in the breast, breast pain or redness and change in nipple structure with discharge.
Trodelvy’s mechanism of action
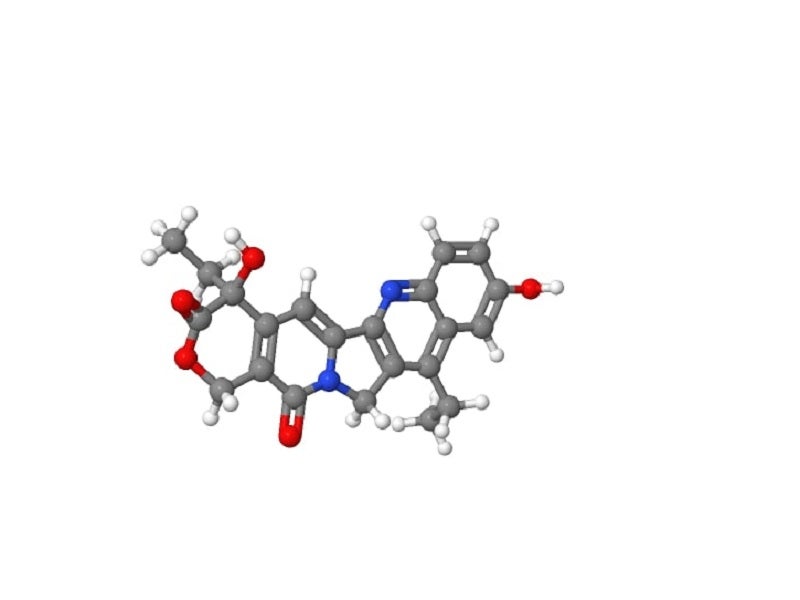
Trodelvy is a Trop-2-directed antibody and topoisomerase inhibitor conjugate drug.
It targets the Trop-2 antigen, a humanised monoclonal antibody, expressed on various tumour cells and delivers SN-38, a topoisomerase I inhibitor, to the tumours directly, causing cell death.
The hydrolysable linker CL2A connects the Trop-2-directed antibody to the cytotoxic drug SN-38.
Trodelvy is currently being studied to address approximately eight difficult-to-treat solid cancers.
Clinical studies of Trodelvy
The FDA’s approval of Trodelvy was based on a multi-centre, single-arm, Phase II clinical trial, IMMU-132-01, which enrolled 108 patients with metastatic TNBC to study the efficacy of the drug. The primary goals of the trial were tumour response rate and duration of response.
In a 21-day treatment cycle, the patients received 10mg/kg of Trodelvy on days one and eight. Tumour imaging was performed every eight weeks with CT or MRI scans for four to six weeks after a partial or complete response. Treatment was continued until disease progression or development of intolerance to the therapy.
Patients demonstrated an overall response rate of 33% in the trial. 30.6% of patients showed partial response and 2.8% of patients showed a complete response, while the median duration of response of 7.7 months was observed in the patients.
The most common adverse events observed in the patients during the IMMU-132-01 clinical trials were diarrhoea, fatigue, nausea, vomiting, baldness, constipation, rashes on the skin, abdominal pain, neutropenia and loss of appetite.
Additional clinical studies on Trodelvy
A phase three confirmatory clinical trial, ASCENT was performed in more than 500 patients with advanced TNBC. The study was halted early due to convincingly powerful efficacy results across multiple parameters.
ASCENT was a global, open-label, randomised Phase III clinical study. The study enrolled patients across 230 study locations and randomised them to receive either Trodelvy or a chemotherapy chosen by the patients’ treating physicians.
The study evaluated the efficacy and safety of Trodelvy compared with a single-agent chemotherapy of the physician’s choice in patients with unresectable, locally advanced or metastatic TNBC who had received at least two prior systemic treatments.
The primary endpoint of the study was progression-free survival (PFS), as determined by blinded independent central review, in patients without brain metastases.
In the study, Trodelvy showed a notable 57% decrease in the risk of disease progression or death, as indicated by PFS, leading to an extension of the median PFS from 1.7 months to 4.8 months compared to chemotherapy. Trodelvy also prolonged the median overall survival (OS) to 11.8 months, as opposed to 6.9 months with chemotherapy, resulting in a 49% reduction in the risk of death.
The most common adverse reactions reported in patients were fatigue, diarrhoea, nausea, alopecia, constipation, vomiting, abdominal pain and reduced appetite.