TRUSELTIQ™ (infigratinib) is an oral kinase inhibitor indicated for the treatment of previously treated, unresectable locally advanced or metastatic cholangiocarcinoma (CCA) with a fibroblast growth factor receptor II (FGFR2) fusion in adult patients.
Infigratinib was developed by QED Therapeutics, a subsidiary of US-based biopharmaceutical company BridgeBio Pharma.
The drug is available as a hard gelatine capsule with a white opaque body in 25mg and 100mg dosage strengths. The 25mg capsule has a grey opaque cap, while the 100mg capsule has a light orange opaque cap.
TRUSELTIQ background and development
In January 2018, BridgeBio Pharma licensed infigratinib from Novartis and established a subsidiary, QED Therapeutics, with $65m of initial financing. QED Therapeutics was founded to advance the development of infigratinib for FGFR-driven disorders.
In December 2018, QED Therapeutics partnered with Foundation Medicine to develop a companion diagnostic for infigratinib.
In March 2021, QED Therapeutics collaborated with Swiss pharmaceutical company Helsinn Group under a licensing agreement worth more than $2bn. The two companies agreed to co-develop, manufacture and commercialise infigratinib worldwide for oncology and other indications, except skeletal dysplasias.
Helsinn Group will be responsible for commercialising the drug outside the US, excluding in China, Hong Kong and Macau, for which LianBio holds the licence.
TRUSELTIQ approvals
In December 2020, the US Food and Drug Administration (FDA) accepted QED Therapeutics’ new drug application (NDA) for infigratinib under priority review designation. The NDA was reviewed under the FDA’s Real-Time Oncology Review (RTOR) pilot programme.
In May 2021, the FDA granted accelerated approval to infigratinib for the treatment of adult patients with cholangiocarcinoma harbouring an FGFR2 fusion or another rearrangement.
The FDA also approved Foundation Medicine’s FoundationOne CDx comprehensive genomic profiling (CGP) test as a registrational companion diagnostic to help select patients with FGFR2 fusion or another rearrangement who could benefit from being treated with infigratinib.
The drug also secured fast track designation and orphan drug designation for the treatment of cholangiocarcinoma.
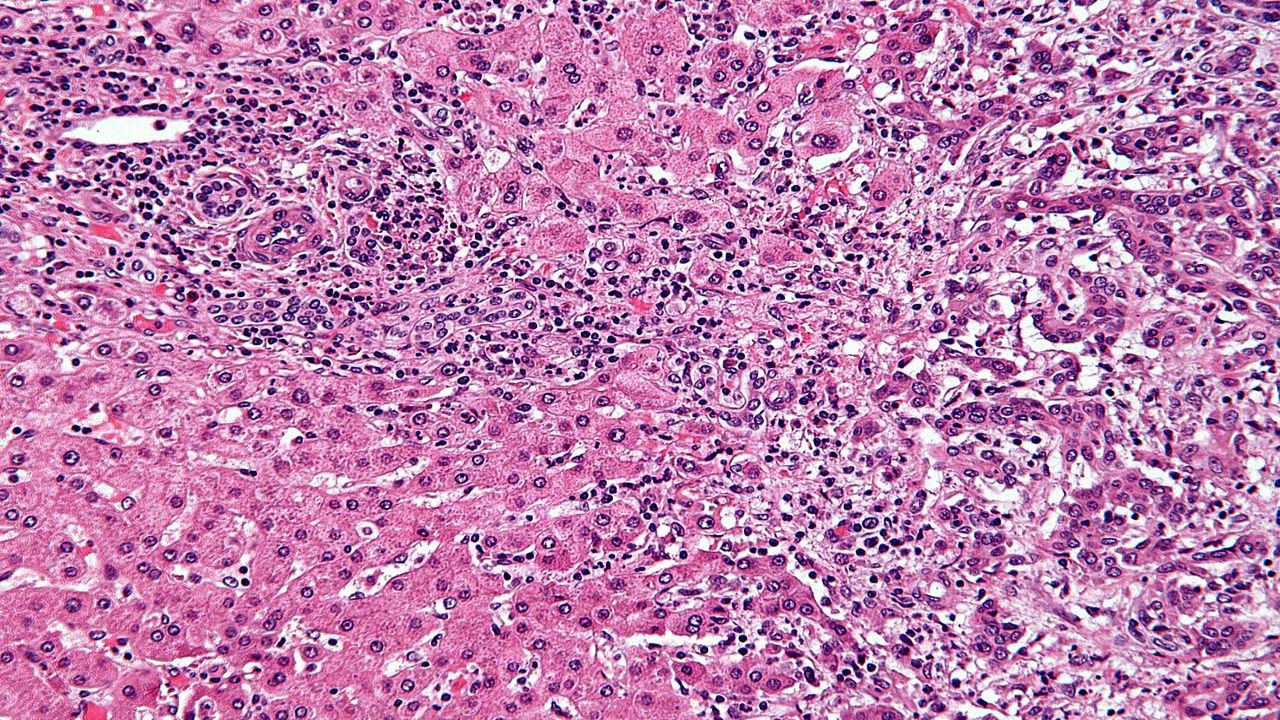
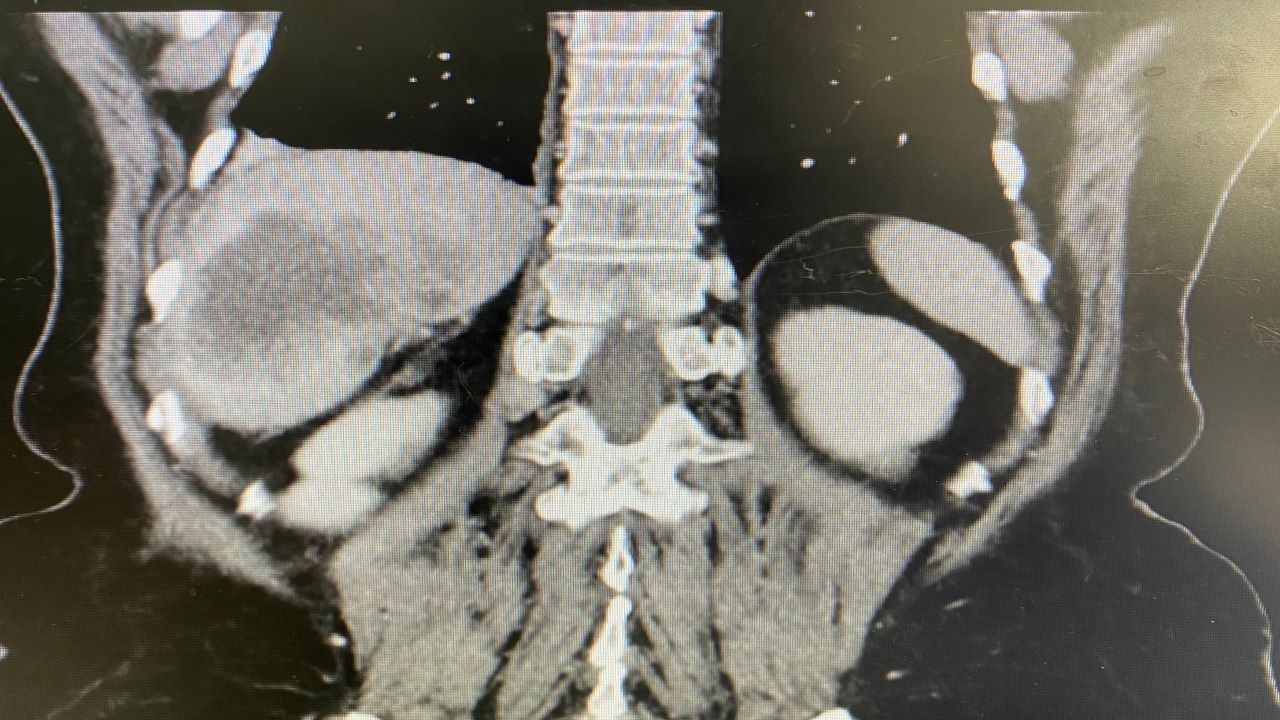
Cholangiocarcinoma causes and symptoms
CCA, also known as bile duct cancer, is a serious and often fatal disease caused by DNA mutations in the cells of bile ducts.
Based on its anatomic location, CCA is classified into three types, namely intrahepatic cholangiocarcinoma, perihilar cholangiocarcinoma and distal cholangiocarcinoma. An estimated 15% to 20% of patients with this condition have FGFR2 genetic abnormalities.
Advanced, unresectable CCA is a rare, aggressive malignancy with a poor prognosis. Signs and symptoms of CCA include yellowing of the skin and the whites of eyes (jaundice), intensely itchy skin, white-coloured stools, fatigue, abdominal pain, and unintended weight loss.
CCA has a median five-year survival rate of only 9%. Around 20,000 people in the US and European Union (EU) are diagnosed with CCA each year.
TRUSELTIQ mechanism of action
TRUSELTIQ is a small-molecule ATP-competitive, tyrosine kinase inhibitor of FGFR. Alterations in FGFR in tumours can lead to constitutive FGFR signalling, supporting the proliferation and survival of malignant cells.
TRUSELTIQ blocks downstream signalling cascades by selectively binding to and suppressing FGFR activities. It induces tumour cell death by decreasing cancer cell proliferation.
Clinical trials on TRUSELTIQ
The FDA’s approval of infigratinib was based on the results of a multi-centre, open-label, single-arm Phase II clinical trial. The study enrolled 108 patients with previously treated, unresectable locally advanced or metastatic CCA with an FGFR2 fusion or rearrangement to evaluate TRUSELTIQ’s efficacy.
The study’s primary efficacy outcome measures were the overall response rate (ORR) and duration of response (DOR), as determined by a blinded independent central review (BICR) according to version 1.1 of the Response Evaluation Criteria in Solid Tumours (RECIST) specification.
In the study, the patients received 125mg of infigratinib once daily for 21 days, followed by a therapy gap of seven days in a 28-day treatment cycle, until either disease progression or unacceptable toxicity.
TRUSELTIQ achieved a 23% ORR, including 1% complete response and 22% partial response. The median DOR in patients was five months.
Common adverse events reported in patients during the clinical study were increased creatinine and phosphate, decreased phosphate, nail toxicity, stomatitis, increased alkaline phosphatase, and decreased haemoglobin.
The TRUSELTIQ-treated patients also experienced increased alanine aminotransferase, dry eye, fatigue, increased lipase, decreased lymphocytes, increased calcium, decreased sodium, alopecia, increased triglycerides and aspartate aminotransferase, decreased platelets, increased urate, palmar-plantar erythrodysesthesia syndrome, arthralgia and dysgeusia.
Serious risks include hyperphosphataemia and retinal pigment epithelial detachment.