Varithena (polidocanol injectable foam) is injectable foam indicated for the treatment of patients with incompetent veins and visible varicosities of the great saphenous vein (GSV) system. The drug was discovered and developed by BTG International.
BTG received approval from the US Food and Drug Administration (FDA) for Varithena for treating patients with varicose veins and saphenofemoral junction incompetence in November 2013.
Varithena is the only FDA-approved comprehensive therapy to improve symptoms and appearance for a wide range of varicose veins. BTG holds the commercial rights of Varithena and plans to launch the drug in the US market in the second quarter of 2014.
Causes and symptoms of varicose veins
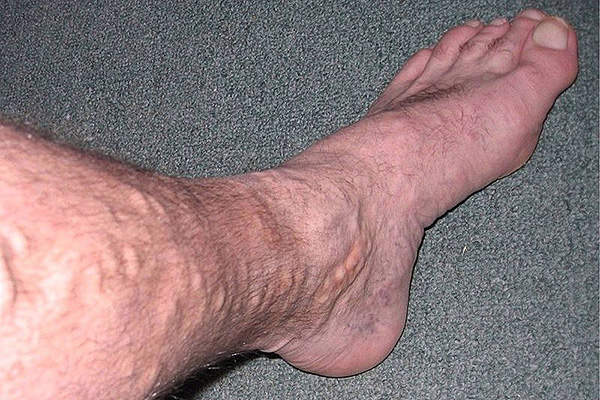
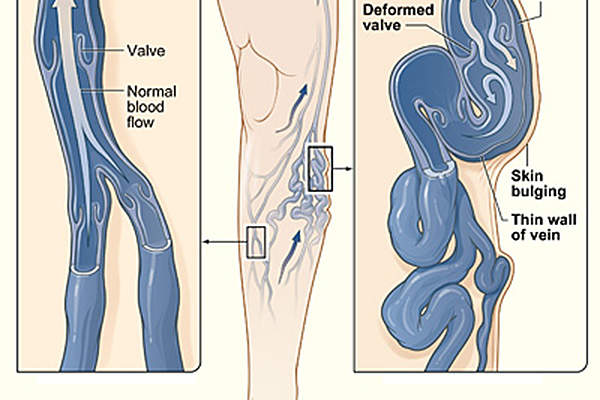
Varicose veins are the superficial, enlarged and tortuous veins found on the legs on the great saphenous vein (GSV) system. The disease is caused by weakened valves and can result in enlarged veins causing aching pain and discomfort.
The symptoms of the disease include heaviness, swelling, aching, restless legs, cramps, itching, throbbing and tingling.
The disease affects about 30million adults in the US and is twice more commonly found in women than in men.
Varithena’s mechanism of action
Varithena contains a sclerosing agent that treats blood vessel malformations. The drug includes polidocanol molecule that connects to the lipid cell membrane of the venous endothelium and disrupts the osmotic barrier, destructs the venous endothelium, and results in vasospasm.
The drug is administered through intravenous injection.
Clinical trials on Varithena
BTG evaluated the safety profile of Varithena by enrolling 1,333 patients in 12 clinical trials including three phase III placebo-controlled, randomised trials. The patients treated with Varithena experienced pain or discomfort in extremity, infusion site thrombosis, injection site hematoma or pain, thrombophlebitis superficial, and extravasation during the clinical trials.
The FDA approval for the drug was based on two phase III clinical trials known as VANISH-1 and VANISH-2. VANISH-1 was conducted between November 2010 and December 2012, whereas VANISH-2 was conducted between November 2010 and June 2012. Both the clinical studies were randomised, blinded and multicentre studies.
VANISH-1 assessed the efficacy and safety of Varithena 0.5%, 1.0% and 2.0%, while VANISH-2 assessed Varithena 0.5% and 1.0%, in comparison with placebo. The two studies enrolled over 519 patients with varicose veins.
The primary endpoint of the studies was improvement in patient symptoms. The secondary endpoint included improvement in appearance in the varisolve arm, and change in appearance as rated by patient and central independent photography review.
The results of the VANISH-1 and VANISH-2 studies demonstrated that the patients administered with Varithena 0.125 were significantly superior to placebo in improving the symptoms. About 64.7% of patients in VANISH-1 study and 75.9% of patients in VANISH-2 study, who were treated with Varithena, showed clinically meaningful improvement in symptoms at week eight.
The clinical findings were supported by the results of duplex ultrasound and other clinical measures.
Marketing commentary
BTG International is involved in the development and commercialisation of products targeting acute care, cancer and vascular diseases.