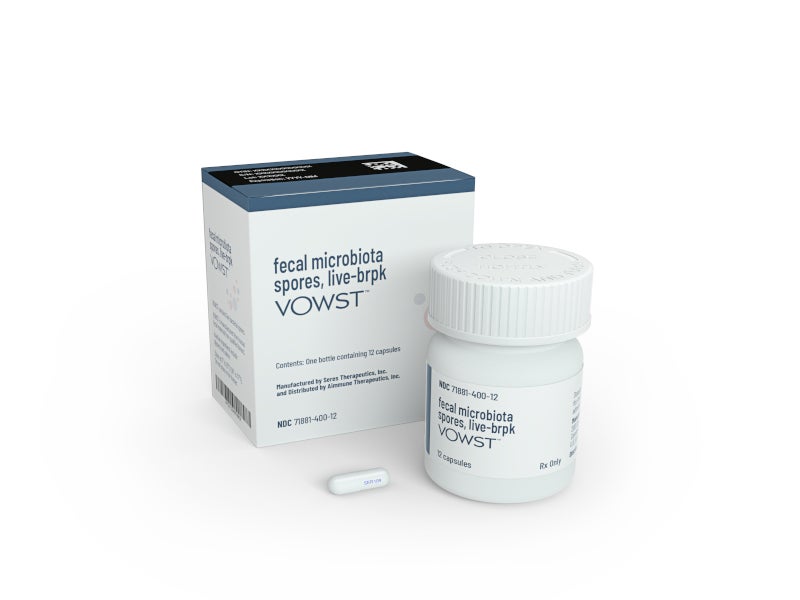
VOWST™ (faecal microbiota spores, live-brpk), formerly known as SER-109, is a microbiome therapeutic indicated to prevent the recurrence of Clostridioides difficile infection (CDI) in people aged 18 years and above after antibacterial treatment for recurrent CDI (rCDI).
Developed by US-based biotech company Seres Therapeutics, VOWST is an orally administered faecal microbiota product consisting of a combination of highly purified Firmicutes spores, which generally live in a healthy microbiome. Each VOWST capsule contains 1×106 to 3×107 Firmicutes spore colony-forming units. A single dose comprises four capsules.
Seres entered an agreement with Nestle Health Science, a healthcare company based in Switzerland, to jointly commercialise VOWST for rCDI in the US and Canada in July 2021. Nestle will be the lead commercialisation party and will utilise its global pharmaceutical business, Aimmune Therapeutics.
Regulatory approvals for VOWST
Seres completed the rolling submission process for the biologics licence application (BLA) for VOWST for the prevention of rCDI to the US Food and Drug Administration (FDA) in September 2022. The FDA accepted the application for review and granted it priority review in October 2022.
The drug also holds breakthrough therapy designation and orphan drug designation for the prevention of rCDI. The FDA approved VOWST for the condition in April 2023.
Clostridium difficile infection: causes and symptoms
Clostridioides difficile, also known as Clostridium difficile or C difficile, is one of the top three most urgent bacterial concerns in the US, according to the Centers for Disease Control and Prevention (CDC).
rCDI is an infection of the gastrointestinal system caused by C difficile bacteria. The human digestive system includes millions of bacteria, which are referred to as the gut flora or gut microbiome. rCDI is connected to dysbiosis of the gut microbiota.
Factors such as taking antibiotics to treat an infection may alter the balance of micro-organisms in the gut. This allows C difficile to multiply and release toxins that can cause diarrhoea, abdominal pain, fever, and even organ failure and death in some cases.
Other risk factors for CDI include being aged over 65, a recent stay in a hospital or a nursing home, having a weaker immune system or having a history of CDI. Symptoms of CDI can include diarrhoea, fever, stomach tenderness or pain, loss of appetite and nausea.
After recovery from CDI, patients may develop the infection again, generally numerous times – a condition known as rCDI. The risk of recurrence increases with each infection, and therapeutic options for rCDI are limited.
VOWST’s mechanism of action
VOWST is a bacterial spore suspension in capsule form for oral administration. It is manufactured from human faecal matter sourced from qualified donors. The donated human faecal matter is routinely tested for a panel of transmissible pathogens.
The spore suspension is created through a process involving the treatment of faecal matter with ethanol to eliminate non-spore organisms. It is followed by filtration stages to eliminate solids and any remaining ethanol.
The mechanism of action of VOWST is yet to be established.
Clinical trials of VOWST
The FDA approval of VOWST was based on a robust Phase III development programme including the ECOSPOR III and ECOSPOR IV clinical studies.
ECOSPOR III was a multi-centre, randomised, placebo-controlled trial on individuals with rCDI. The primary objective of the study was to demonstrate the reduction of CDI recurrence with VOWST.
A total of 349 patients with confirmed rCDI were randomised (1:1) to receive either VOWST or placebo (capsules containing 92 4% glycerol in saline) for three consecutive days.
In the study, VOWST reduced CDI recurrence at eight weeks with approximately 88% of patients recurrence-free at eight weeks post-treatment, compared to 60% in placebo participants.
Additionally, at six months post-treatment, 79% of the patients in the VOWST group were recurrence-free, compared to 53% in the placebo group.
The most common adverse events observed in the patients during the clinical trial were abdominal distention, fatigue, constipation, chills and diarrhoea.
ECOSPOR IV was an open-label, single-arm study that evaluated VOWST in 263 adults with rCDI.
The study was conducted to evaluate the safety and rate of CDI recurrence after administration of VOWST through 24 weeks.
In the study, the drug was well-tolerated, and the overall rate of recurrent CDI was low, consistent with the ECOSPOR III placebo-controlled randomised clinical trial. The study demonstrated the superiority of VOWST compared with placebo in reducing CDI recurrence rates.