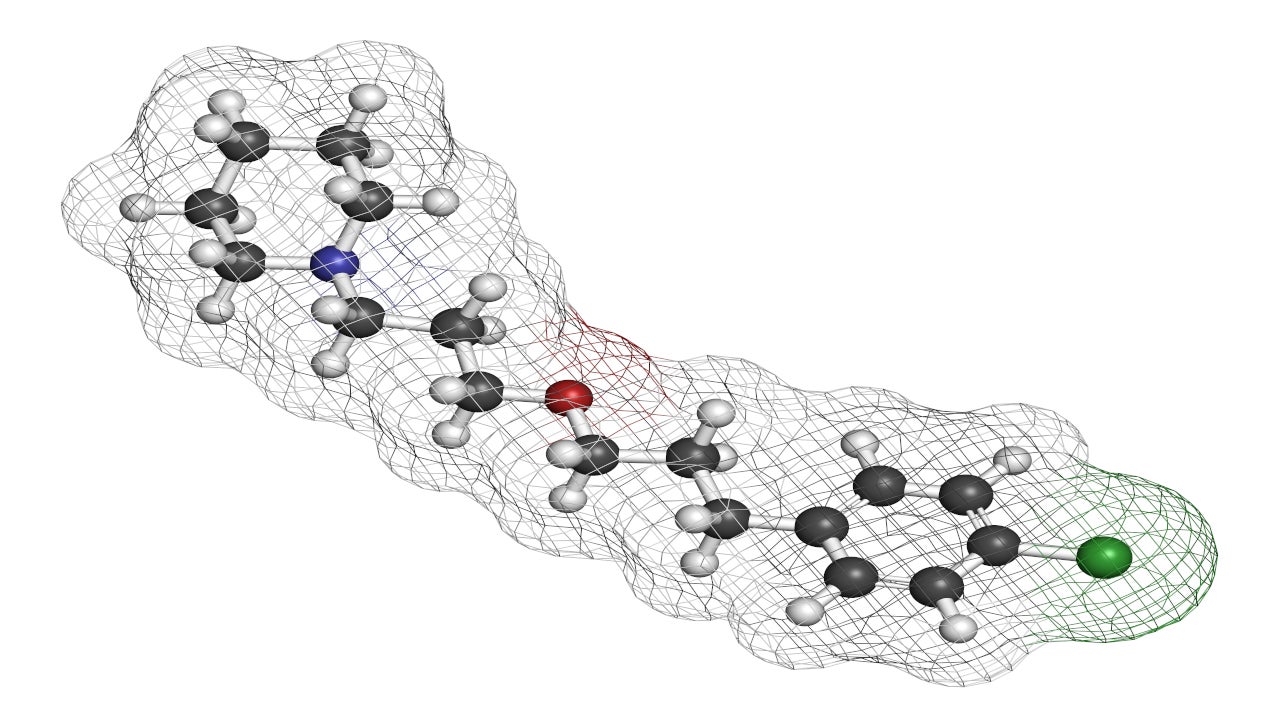
WAKIX® (pitolisant) is the first and only non-scheduled medication indicated for the treatment of cataplexy or excessive daytime sleepiness (EDS) in adult patients with narcolepsy.
The drug is available as white, round, biconvex film-coated tablets in 4.45mg and 17.8mg strengths, for oral administration.
WAKIX approvals
Designed and developed by Bioprojet Societe Civile de Recherche (Bioprojet), WAKIX was granted orphan designation for narcolepsy in Europe in 2007 and approved for narcolepsy with or without cataplexy as an unscheduled drug by the European Medicines Agency (EMA) in March 2016.
The drug is marketed or distributed under a compassionate use programme in nine countries in the European Union (EU), including Germany, France, Netherlands, Italy, Belgium, Ireland, UK, Switzerland and Spain.
Harmony holds an exclusive authorisation from Bioprojet for the development, manufacturing and commercialisation of pitolisant in the US.
The US Food and Drug Administration (FDA) provided orphan designation to pitolisant for narcolepsy in 2010, while breakthrough therapy designation to the drug for cataplexy in patients with narcolepsy was granted in April 2018.
Harmony submitted an original new drug application (NDA) for pitolisant to the FDA for the treatment of EDS and cataplexy in adult patients with narcolepsy in December 2018 and the application was accepted for review in February 2019.
The FDA approved WAKIX (pitolisant) for the treatment of EDS in adult patients with narcolepsy in August 2019 and extended the label for the treatment of cataplexy in adult narcolepsy patients in October 2020.
Cataplexy causes and symptoms
Cataplexy is one of the physical manifestations of narcolepsy, the most debilitating chronic, rare neurological condition, characterised by abrupt, transient lack of muscle tone, often induced by strong feelings such as excitement or laughter.
It is an attribution of REM sleep dysregulation, causing wakefulness. It ranges from moderate symptoms, such as drooping of eyelids, to severe, such as buckling of the knee or complete collapse of the body.
Cataplexy may have a profound effect on the ability of a person to perform normal functions. All narcolepsy patients show excessive daytime sleepiness, while approximately two-thirds of them experience catalepsy.
Pitolisant mechanism of action
Pitolisant is a potent and highly selective antagonist or inverse agonist of the histamine-3 (H3) receptor.
The mechanism of action of WAKIX is unclear, however, its efficacy can be established through its activity on H3 receptors. WAKIX binds to H3 receptors selectively and increases synthesis and release of histamine, a neurotransmitter that promotes wakefulness in the brain.
Clinical trials on WAKIX
FDA approval of WAKIX was based on the two multi-centre, randomised, double-blind, placebo-controlled trials, HARMONY CTP and HARMONY 1.
Harmony CTP registered patients aged 18 years and above who met the International Classification of Sleep Disorders (ICSD-2) criteria for narcolepsy with cataplexy with at least three cataplexy attacks in a week. Patients meeting the ICSD-2 criteria for narcolepsy (with or without cataplexy) and an ESS score of fourteen or more enrolled in Harmony 1.
HARMONY CTP study included a seven-week treatment with a three-week dose-titration phase, followed by a four-week stable dose phase.
105 patients were randomised to receive an initial dose of 4.45mg once daily for the first week along with an initial titration dose of 8.9mg for the second week, increasing to a maximum of 35.6mg at the next two-week intervals depending on clinical response and tolerability.
The primary endpoint was the final mean weekly cataplexy rate over the four weeks stable dosing period (adjusted for baseline differences).WAKIX showed a statistically significant improvement on the primary endpoint compared to placebo.
The primary adverse reactions reported during the clinical trials were insomnia, nausea and anxiety.