Yervoy is a monoclonal antibody drug indicated for treating metastatic melanoma. The drug was developed by Bristol-Myers Squibb.
In March 2011, The US Food and Drug Administration (FDA) approved Yervoy to treat patients with newly diagnosed or previously-treated unresectable or metastatic melanoma. Yervoy is the first drug approved vor the treatment of metastatic melanoma in the US.
Bristol-Myers Squibb submitted a marketing authorisation application to the European Medicines Agency in May 2010. The drug received approval from the European Commission in July 2011.
Approval from Australia’s Therapeutic Goods Association was received in July 2011. The drug is currently being reviewed by Health Canada.
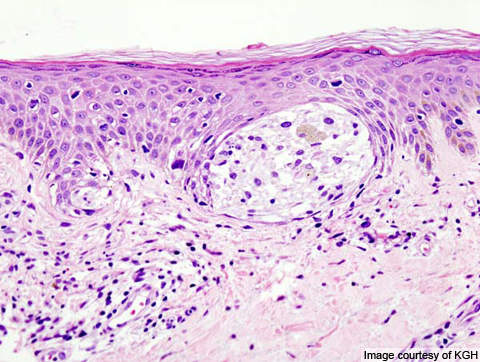
Metastatic melanoma
Melanoma responsible for majority of skin cancer deaths in the US. In metastatic melanoma the cancer spreads to other parts of the body from its starting point. It becomes difficult to treat the disease once it spreads beyond the skin to other parts of the body. The disease is also known as stage IV melanoma.
If the melanoma spreads to the lungs then the patient faces breathing problems. The patients with metastatic melanoma may feel symptoms of fatigue, loss of weight, and appetite and bowel problems.
The incidence of the disease has increased steadily in the US after 1970s. The American Cancer Society (ACS) estimated that more than 68,000 new cases of melanoma were registered in the US in 2009. The ACS estimated that the number of deaths occurred due to melanoma in 2010 was more than 8,700.
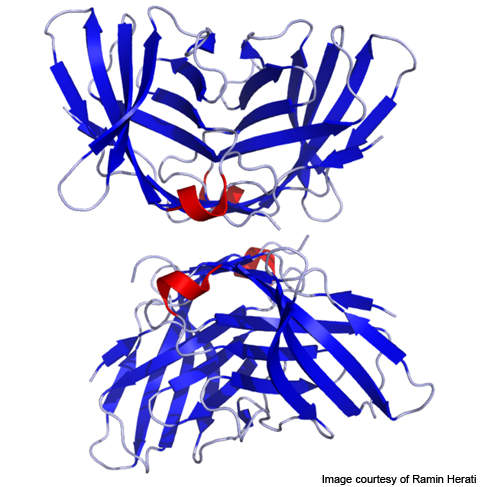
Yervoy mechanism
Yervoy treats metastatic melanoma by activating the immune system. The drug works by binding or inhibiting cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), a molecule that plays vital role in relating natural immune responses. The presence or absence of CTLA-4 can curb or increase the immune system’s T-cell response in fighting disease.
The drug also works by blocking a complex set of interactions in the immune system. It is designed to inhibit the activity of CTLA-4, thereby sustaining an active immune response in its attack on cancer cells.
Clinical trials
BMS has conducted multicentre phase I clinical trials on Yervoy by recruiting 33 advanced melanoma patients. The study found that the drug stimulated the body’s own immunity system to fight melanoma by inhibiting the CTLA-4 molecule. Seven out of 33 participants had experienced PSA declines of 50% during the study.
Three separate phase II clinical trials on the drug were conducted in the US. All the phase II trials demonstrated that using the drug for treatment of advanced melanoma results in a one-year survival rate of about 47% to 51%. The two-year survival rate in patients who received Yervoy dose of 10mg/kg during the trials ranged from 29.8% to 41.8%.
In June 2008, BMS started phase III clinical trials on Yervoy. The final results of the study are expected to come by September 2014. The study will recruit 950 stage III Melanoma patients and will focus on finding the efficacy of Yervoy in comparison with a dummy treatment or placebo. It will also establish whether administering Yervoy improves the recurrence-free survival (RFS) when compared to placebo.
The FDA approval for Yervoy was based on the double-blind and randomised phase III clinical trials (known as MDX010-020 trial) conducted on 676 patients. The primary endpoint of the study was finding the overall survival in Yervoy plus gp100 arm compared to gp100 arm. The secondary endpoints included finding the overall survival in the Yervoy arm versus gp100 arm at week 24 and the best overall response rate.
The patients were administered with Yervoy 3mg/kg through intravenous infusion for more than 90 minutes every three weeks. The assessment of tumours was conducted at weeks 12 and 24 initially and thereafter for every three months. Patients who were treated with Yervoy achieved an overall survival of 10 months compared to six months in gp100 arm.
Phase III trials were also initiated in squamous non small cell lung cancer and prostrate cancer patients in June 2010.
The first trial will evaluate the efficacy of the drug in prostrate cancer patients who have not received prior chemotherapy. Around 600 patients have been enrolled for this trial, which is expected to be completed by November 2015.
The second trial, initiated in August 2011, will compare the efficacy of ipilimumab in combination with paclitaxel and carboplatin compared to a placebo and these two drugs.
It is being conducted in patients with squamous non small cell lung cancer. Around 900 patients have been enrolled in this trial, which is expected to be completed by June 2016. Primary completion is expected in September 2014.
Marketing commentary
The incidence of advanced melanoma in the US is increasing by 3-5% a year. The current therapies have so far not significantly improved the survival rate in melanoma patients. Yervoy is the first drug approved for treating this indication. The FDA has not approved any new first-line medicines in over ten years.
A dosage of Yervoy is expected to cost about $30,000; therapy of four infusions over three months will cost around $120,000. The drug is estimated to have a market of more than $400m in 2011.