Zepzelca™ (lurbinectedin) is a prescription medicine indicated for the treatment of adult patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
Developed by PharmaMar and Jazz Pharmaceuticals, the new drug application (NDA) for Zepzelca was submitted to the US Food and Drug Administration (FDA) in December 2019 and accepted for review in February 2020.
The FDA approved Zepzelca for the treatment of metastatic SCLC with disease progression on or after platinum-based chemotherapy in June 2020 ahead of the Prescription Drug User Fee Act (PDUFA) target date of 16 August 2020. Zepzelca was granted orphan drug designation for the treatment of SCLC by the FDA in August 2018.
PharmaMar and Jazz Pharmaceuticals entered into a license agreement in December 2019 granting marketing rights of Zepzelca in the US to the latter. PharmaMar retains development rights for the drug and will supply Jazz with the product.
Zepzelca is available as an injection administered intravenously with a recommended dosage of 3.2mg / m2 every 21 days. The drug is expected to be available in the US at the beginning of July 2020.
Small cell lung cancer causes and symptoms
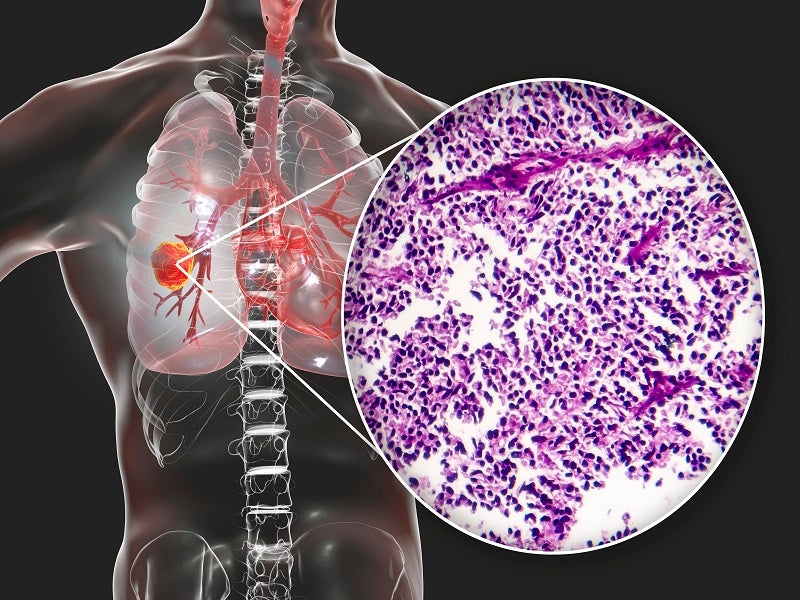
SCLC is a form of lung cancer that grows much faster than non-small cell lung cancer, which normally starts in the middle of the chest at the breathing tubes (bronchi) with the formation of tumours. Although the tumour cells are initially small, they grow rapidly and form huge tumours that spread quickly or metastasis to other areas of the body, including the brain, liver, and bone.
Approximately 10%-15% of all lung cancer cases are SCLC. The condition is significantly more common in males than in females and majorly caused due to cigarette smoking.
Symptoms of SCLC include chest pain, weakness, hoarseness or changing voice, worsening cough, difficulty swallowing, facial swelling, bloody sputum (phlegm), loss of appetite, wheezing, shortness of breath, weight loss and fever.
Lurbinectedin mechanism of action
Lurbinectedin is an alkylating agent that attaches to the guanine residues in the lower groove of the DNA resulting in the formation of conjugates or adducts and bending of the DNA helix towards the main groove.
The formation of adducts leads to a cascade of events, including DNA binding, transcription factors and DNA repair pathways, which interfere with the cell cycle and result in cell death.
Lurbinectedin was also effective in blocking human monocyte activity in vitro and decreased the penetration of the macrophage in implanted tumours in mice.
Clinical trials on Zepzelca
FDA approval of Zepzelca is based on the multicentre, open-label, multi-cohort, single-arm, phase II monotherapy clinical trial Study B-005.
The study enrolled a total of 105 metastatic SCLC patients with progression of disease on or after platinum-based chemotherapy treatment. The patients were administered with lurbinectedin 3.2mg / m2 every 21 days (one cycle) until disease progression or unacceptable toxicity.
A median of four Zepzelca cycles (range one to 24) was given to patients. Tumour evaluations were conducted every six weeks for the first 18 weeks and every nine weeks subsequently.
The primary efficacy outcome measure was confirmed investigator-assessed overall response rate (ORR). Additional efficacy outcome measures included duration of response (DoR) and an Independent Review Committee (IRC) assessed ORR using RECIST v1.1.
ORR was 35% with a median response duration of 5.3 months as measured by the investigator assessment, while ORR as per the independent review committee was 30% with a mean DoR of 5.1 months.
The most common adverse reactions observed during the trial were myelosuppression, increased levels of creatinine and alanine aminotransferase, laboratory abnormalities, musculoskeletal pain, decreased appetite, nausea, fatigue, decreased albumin, and increased glucose.