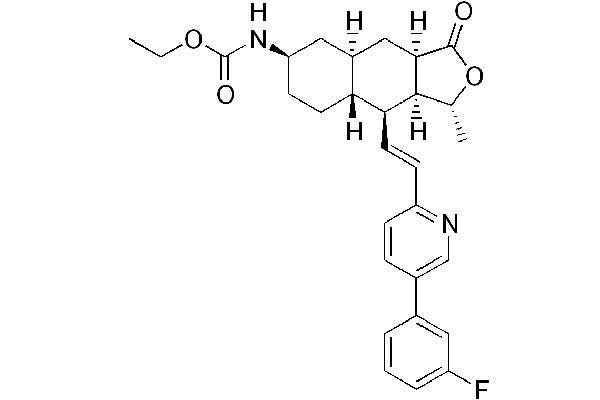
Zontivity (vorapaxar) is a protease-activated receptor-1 (PAR-1) antagonist indicated for the reduction of thrombotic cardiovascular events in patients with a history of heart attack or peripheral arterial disease (PAD). The drug was discovered by Schering-Plough and developed by Merck.
In May 2014, the US Food and Drug Administration (FDA) granted approval for Zontivity for the reduction of thrombotic cardiovascular events in patients with a history of heart attack or in patients with narrowing of leg arteries, called PAD.
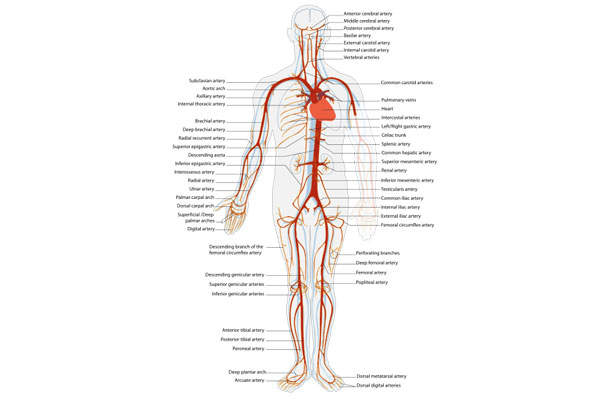
Heart attack and peripheral arterial disease (PAD) treatment
An investigational therapeutic agent being developed for treating patients with acute decompensated heart failure.
A heart attack is caused by blood clot or thrombus formation in a coronary artery. It is estimated that about 7.6 million people in the US have survived a heart attack. It is also observed that around 515,000 new and 205,000 recurrent cases of heart attack are registered in the US annually.
Peripheral arterial disease (PAD) occurs when arteries that carry blood to head, organs and limbs are blocked. The disease affects approximately 8.5 million people in the US and PAD patients are found to be at increased risk for heart attack, stroke and cardiovascular death.
Zontivity mechanism of action
Zontivity contains vorapaxar sulphate, a protease-activated receptor-1 (PAR-1) antagonist, which inhibits platelet aggregation mediated by PAR-1. The drug restores blood flow to the heart by decreasing the formation of blood clots in patients with previous heart attack or blockages in the arteries to the legs.
The drug is available in tablet form for oral administration.
Clinical trials of Zontivity
FDA approval for Zontivity was based on the pivotal TRA 2°P TIMI 50 (‘Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events’) trial. The clinical study enrolled 26,449 patients with history of spontaneous myocardial infarctions (MI) within the prior two weeks to 12 months, ischemic stroke, or documented (symptomatic) PAD. It was a randomised, double-blind, placebo-controlled clinical trial.
Patients were randomised to receive Zontivity daily with standard of care that included aspirin and/or a thienopyridine (principally clopidogrel), or standard of care alone. Patients followed for up to four years, with a median follow-up of 2.5 years.
Results demonstrated that patients treated with Zontivity daily with a standard of care witnessed superior reduction in the incidence of both the primary and secondary combined endpoints of cardiovascular (CV) death, MI, and a stroke.
The efficacy and safety of Zontivity was evaluated in the overall study population. A total of 10,080 patients were randomised to treatment with Zontivity, and 10,090 were randomised to receive placebo. Patients included high-risk diabetics and hypertensives.
Results from the study showed that the patients treated with Zontivity achieved 17% relative risk reduction through three years for the composite primary efficacy endpoint of CV death, MI, stroke, and urgent coronary revascularisation (UCR). The patients administered with Zontivity achieved 20% relative risk reduction through three years for the key secondary composite efficacy endpoint of CV death, MI, and stroke.
Adverse reactions found in patients treated with Zontivity included an increased rate of GUSTO moderate or severe bleeding. The GUSTO moderate or severe bleeding in the Zontivity group through three years was 3.7% compared with 2.4% in placebo. GUSTO severe bleeding occurred in Zontivity group at a rate of 1.3% versus 1.0% for placebo.
Marketing commentary
Merck plans to launch Zontivity in the US market in the third quarter of 2014. A similar drug available in the market for same indication is Xarelto / rivaroxaban developed by Bayer and Johnson & Johnson.