Zydelig (idelalisib) is a phosphoinositide 3-kinase (PI3K) delta inhibitor and is used for the treatment of three B-cell blood cancers: relapsed chronic lymphocytic leukaemia (CLL), follicular lymphoma (FL) and small lymphocytic lymphoma (SLL). The drug is developed and marketed by Gilead Sciences.
The US Food and Drug Administration (FDA) approved Zydelig (idelalisib) for the treatment of CLL, FL and SLL in July 2014.
Gilead Sciences received a positive opinion on Zydelig’s marketing authorisation application (MAA) from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for the treatment of CLL and FL in July 2014. The drug obtained marketing authorisation in September 2014.
The EMA’s approval was for Zydelig combined with rituximab for CLL patients who received at least one prior therapy, or as first-line treatment in the presence of 17p deletion or TP53 mutation for patients not suitable for chemo-immunotherapy. The drug has also been approved as a monotherapy for FL patients who are refractory to two prior lines of treatment.
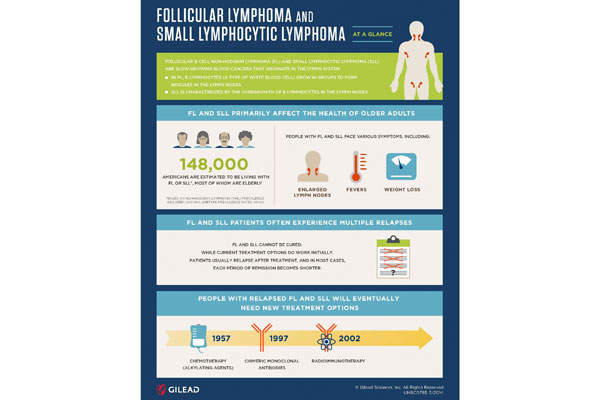
The three B-cell blood cancers
CLL is a blood cancer in which the bone marrow overproduces B-lymphocytes. Its symptoms include fatigue, bleeding, anaemia and infections. It is estimated that 120,000 people in the US are affected by CLL, and 4,500 people die from it each year.
FL and SLL are two types of blood cancers that grow at a slow pace in the lymph system. FL occurs when B-lymphocytes grow in groups to form nodules, while SLL occurs when the B-lymphocytes overgrow in the lymph nodes. The symptoms of FL and SLL include high fever, weight loss and enlarged lymph nodes. It is estimated that 148,000 people in the US are living with FL and SLL.
Zydelig’s mechanism of action
Zydelig inhibits phosphoinositide 3-kinase (PI3K) delta, a main protein involved in the activation, viability, proliferation and migration of B-cells. The drug works by blocking the pathways that cause B-cell viability.
Zydelig is available in the form of 100mg and 150mg-dosed tablets for oral administration.
Clinical trials on Zydelig
The FDA approval of Zydelig for the treatment of CLL patients was based on the results obtained from a Phase III clinical trial known as Study 116. The CHMP gave a positive opinion on Zydelig based on the same clinical trial.
Related content
Arzerra (Ofatumumab) for Treatment of Chronic Lymphocytic Leukaemia (CLL), USA
Arzerra (ofatumumab) is the first-line drug approved for the treatment of chronic lymphocytic leukaemia (CLL). The drug is developed and marketed by GlaxoSmithKline (GSK) in collaboration with Genmab.
Study 116 was a randomised and placebo-controlled clinical study. It enrolled 220 patients with relapsed CLL for whom standard chemotherapy was not suitable. One set of the patients was treated with Zydelig and rituximab while the others received rituximab alone.
However, the study was ended by the Data Monitoring Committee in October 2013 due to the statistically significant benefit in progression-free survival (PFS) in the Zydelig arm in comparison to the placebo plus rituximab arm. The study also revealed that the median PFS was not reached in the Zydelig plus rituximab arm, while it was five and a half months in the placebo plus rituximab arm.
The FDA granted accelerated approval for Zydelig for the treatment of FL and SLL based on the data received from a Phase II single-arm study named Study 101-09. The study was conducted on patients for whom Zydelig was considered as monotherapy, as well as those who are refractory to rituximab and alkylating agent containing chemotherapy.
The study results demonstrated that Zydelig achieved an overall response rate of 54% in FL patients and 58% in SLL patients. The most common adverse reactions found in the Zydelig-administered patients included pyrexia, diarrhoea, fatigue, cough, nausea, abdominal pain, chills and rash. The most frequent serious adverse reactions recorded in the combination were pneumonia, sepsis, pyrexia, febrile neutropenia and diarrhoea.
The most common lab abnormalities included hypertriglyceridemia, neutropenia and hyperglycaemia.
Marketing commentary
Based in Foster City, California, Gilead Sciences is a biopharmaceutical company that focuses on the discovery, production and marketing of innovative therapeutics in areas of unmet medical need.
Other medications available in the market for the treatment of these cancers include Arzerra (Ofatumumab) manufactured by GlaxoSmithKline (GSK) and Genmab and Gazyva (obinutuzumab) developed by Roche and Biogen Idec.