With the increase in logistical and recruitment challenges for conducting clinical trials amid the COVID-19 pandemic, the pharmaceutical and healthcare industry started switching to virtual or decentralised clinical trial programmes, says GlobalData.
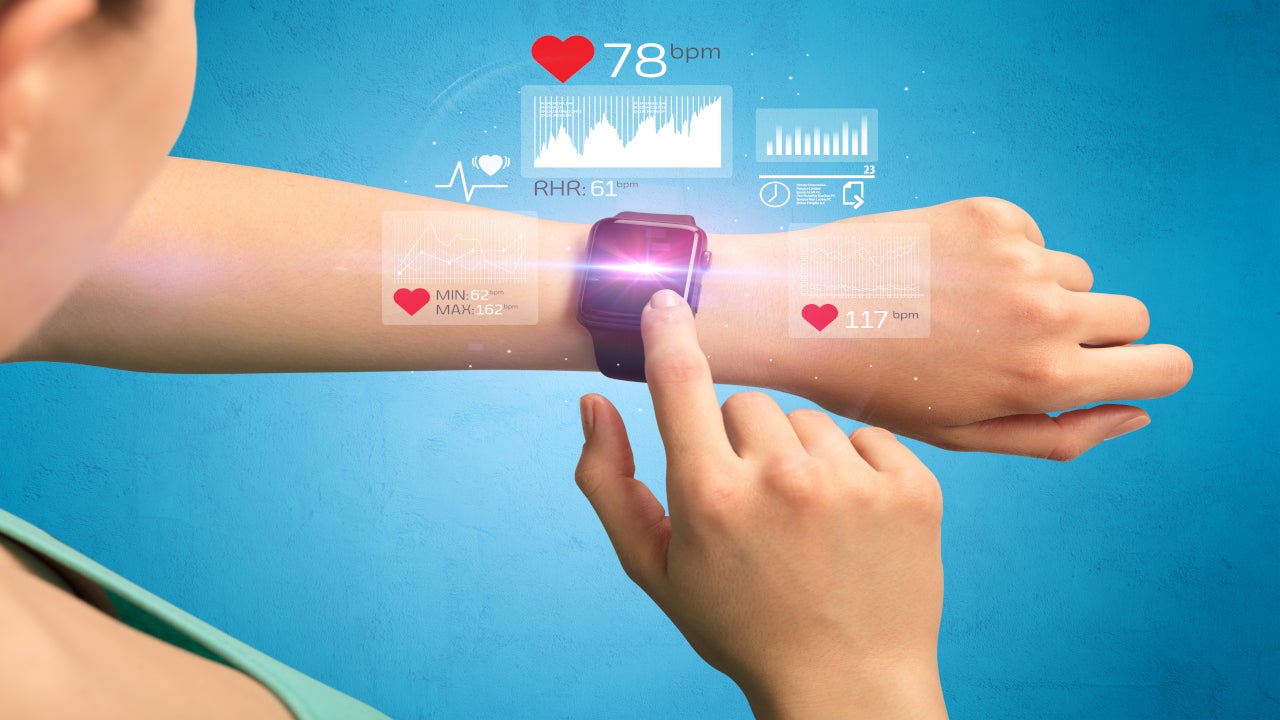
Virtual or decentralised trials use digital tools such as wearables to enable remote monitoring of the patients. The digital tools use connected devices to monitor and track different physiological data from vital signs such as heart rate, respiration rate and oxygen saturation enabling patients to participate from their own homes.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
These devices also enable tracking of other parameters such as continuous glucose monitoring (CGM) and sleep and activity data to procure necessary health information from the patients.
Such remote patient monitoring (RPM) strategies help in eliminating the need for patients to travel to trial sites thereby ensuring compliance with social distancing measures. RPM is expected to particularly benefit those clinical trial participants who suffer from serious underlying health conditions and may rely on experimental medicines.
The virtual trials may also prove potentially less costlier compared to conducting such programmes in traditional settings. Further, the suspension of trials altogether, at the time of the pandemic, may leave many of these patients without treatment options.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData




