Since Novartis’ CTL019 received unanimous recommendation for approval in acute lymphoblastic leukemia (ALL) by the US Food and Drug Administration (FDA) advisory committee on the 12th of July 2017, the development of chimeric antigen receptors T cell (CAR-T) therapies has been watched carefully to anticipate the clinical and commercial impact of these immuno-oncology agents.
Another class of passive immuno-oncology agents, bispecific antibodies received their first FDA approval for Blincyto (blinatumomab) in December 2014. Although these two therapeutic classes aim at redirecting T-cells toward tumour cells, they present major differences in terms of structure and efficacy / safety profile, and CAR-T cells also require a more cumbersome manufacturing procedure.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
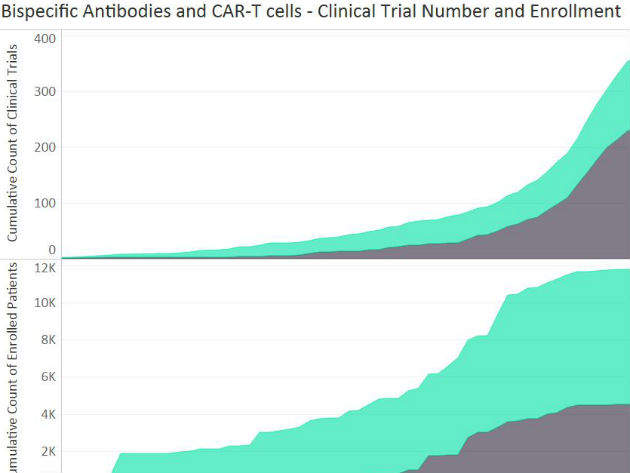
As presented in the figure below, when comparing the number of trials with CAR-T cells and bispecific antibodies, CAR-T cell-based trials have been increasing at a much higher rate than bispecific antibody-based trials since 2004. In Q2 2017, there were almost two times more clinical trials for CAR-T cells than for bispecific antibodies.

However, when comparing enrolment into clinical trials assessing bispecific antibodies and CAR-T cells, the number of patients enrolled was much higher for bispecific antibodies.
According to GlobalData’s analysis, most trials evaluating bispecific antibodies are sponsored by companies, while CAR-T cell trials are mostly run by institutions. As companies are responsible for most of the patient recruitment into clinical trials, it remains higher for bispecific antibodies.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThis trend could be challenged by the future development of allogenic CAR-T cells allowing for an easier manufacturing process and the acquisition of such cell-based technologies by pharmaceutical companies.





