No Filter Selected
No Filter Selected
No Filter Selected
No Filter Selected
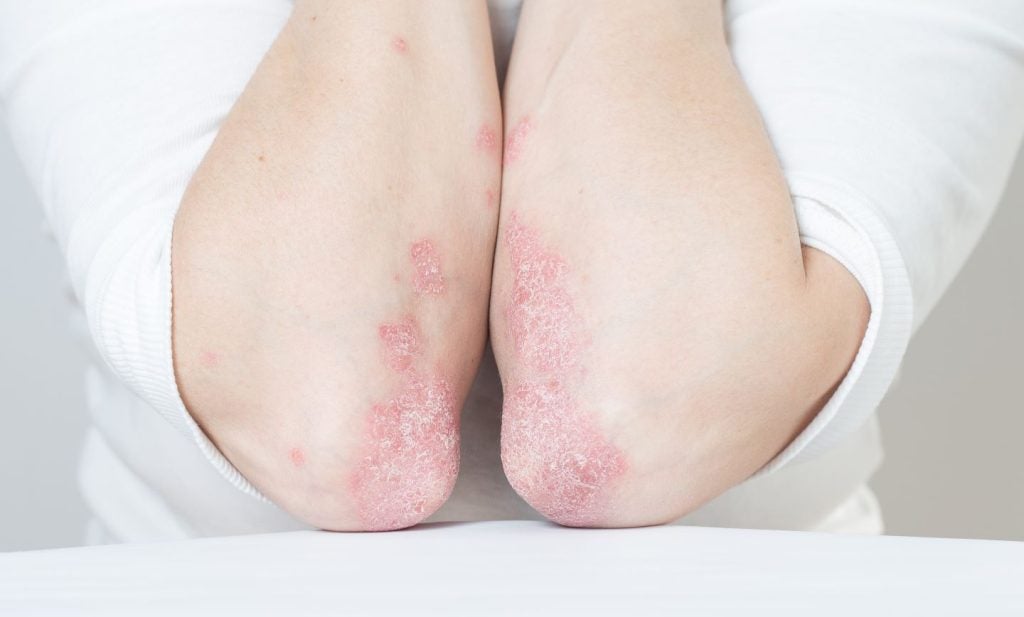
LEO Pharma reports positive data from Phase III plaque psoriasis trial
Enstilar demonstrated superiority in both primary and secondary endpoints of the trial.
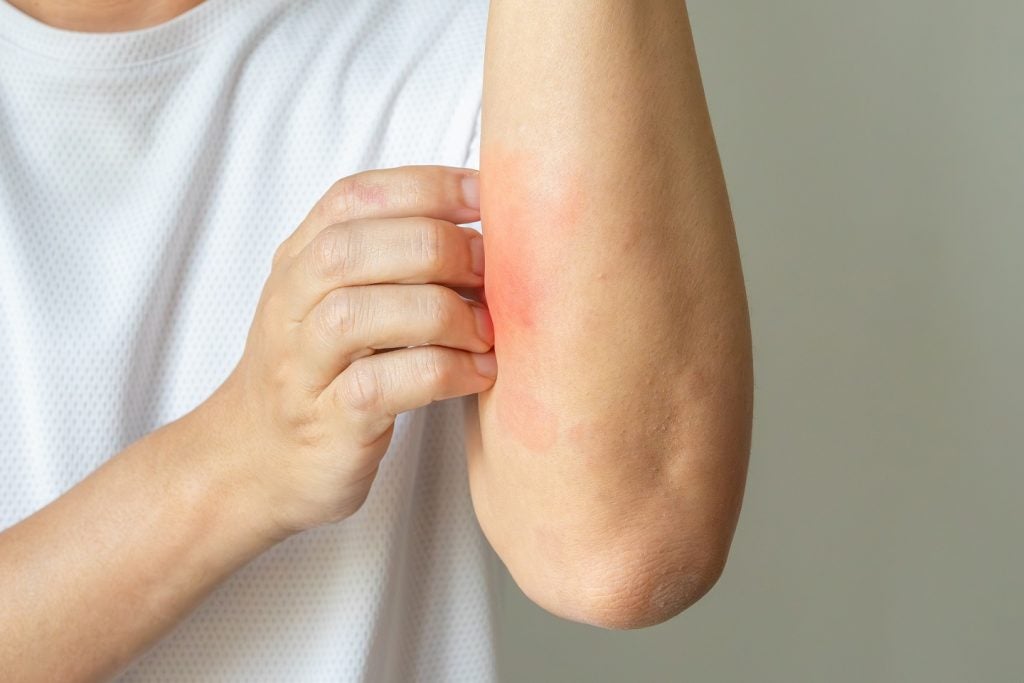
LEO Pharma reports outcomes from Phase IIb trial of atopic dermatitis therapy
Subjects in the trial were randomised and were given one of four doses of the therapy or a placebo.

Leo Pharma’s Timber bet falls flat following Phase III failure
Leo Pharma spent $36m to acquire Timber Pharmaceuticals last year, along with its lead dermatology asset TMB-001.

LEO Pharma and ICON partner for clinical trial execution in dermatology
The collaboration is expected to provide access to innovative clinical trials as well as help to launch new medicines.
Leo Pharma partners with Veeva Systems to digitalise clinical trials
LEO Pharma will use Veeva’s CDMS, eConsent, eTMF, CTMS, Site Connect, ePRO, Virtual Visits, and eSource products.

JPM26: Leo Pharma’s growth strategy puts a spotlight on rare dermatology diseases
Advancements in the form of recent approvals and an evolving pipeline landscape in dermatology will bolster Leo’s chance of success.
Deals this week: Affimed, LEO Pharma, Harbour BioMed
Affimed has entered an agreement with Roche Group’s subsidiary Genentech for the development and commercialisation of immuno-therapeutics for multiple cancer targets.
Deals this week: Kazia Therapeutics, Zymeworks, CANbridge
Kazia Therapeutics has signed a collaboration agreement with Dana-Farber Cancer Institute to trial GDC-0084 for the treatment of patients diagnosed with breast cancer that has spread to the brain.
Deals this week: HitGen, Calithera Biosciences, Esperion Therapeutics
HitGen has signed an agreement with LEO Pharma to use its advanced technology platform to discover new small molecules.

LEO Pharma reports positive data from eczema therapy trial
The long-term safety profile of delgocitinib cream was in line with that recorded in prior data from DELTA 1 and 2 trials.
Deals this week: Roivant Sciences, Inflazome, Santhera
Roivant Sciences will use biologic drug candidate SAL200 are entering a global licensing agreement worth $667.5m with iNtRON Biotechnology.
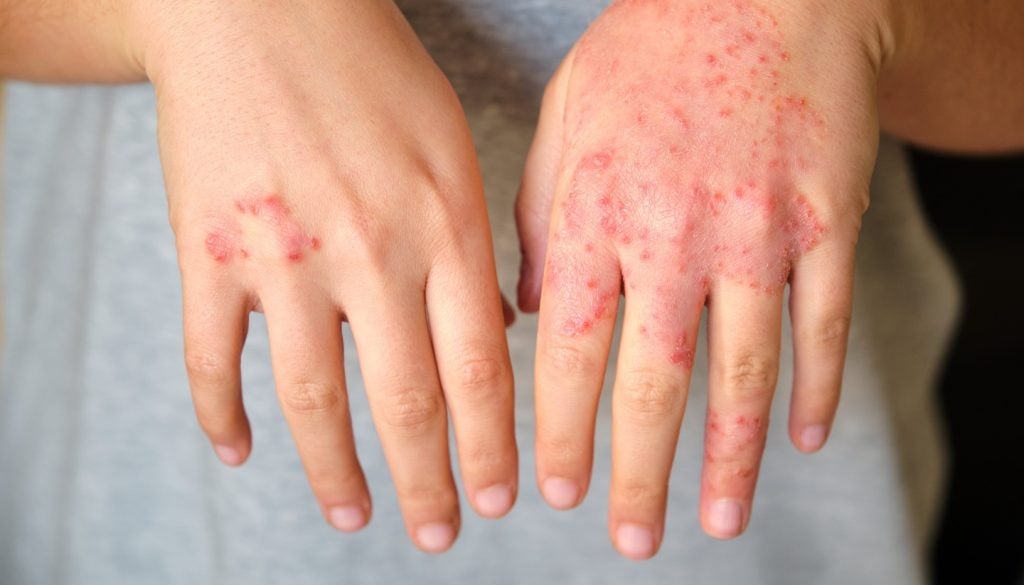
LEO Pharma reports results from interim analysis of Phase IIIb atopic dermatitis trial
The trial aims to assess the safety and efficacy of tralokinumab 300mg administered biweekly as a single agent.

Leo Pharma's Daniel Ramirez on recruitment success, remote technology, and the clinical trials industry
Daniel Ramirez, VP of technology, search and partnering at Leo Pharma, discusses his upcoming presentation at Outsourcing in Clinical Trials...
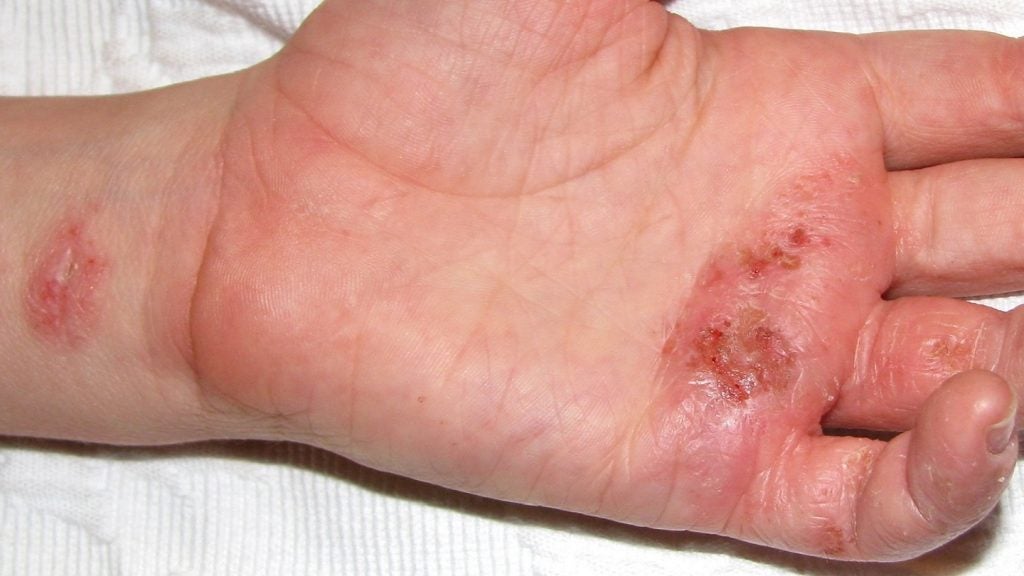
LEO Pharma reports positive data from trial of chronic hand eczema therapy
The trial assessed the efficacy of twice daily delgocitinib cream to treat moderate to severe CHE.
Deals this week: miRagen Therapeutics, Zhongyuan Union Cell, Health Advance
US-based biopharmaceutical company miRagen Therapeutics plans to raise $40m through private placement of its common stock shares with the help of placement agent Wedbush PacGrow.
LEO Pharma’s tralokinumab meets all primary and secondary endpoints in three pivotal trials
Anti-interleukin-13 is in development for the treatment of moderate-to-severe atopic dermatitis.
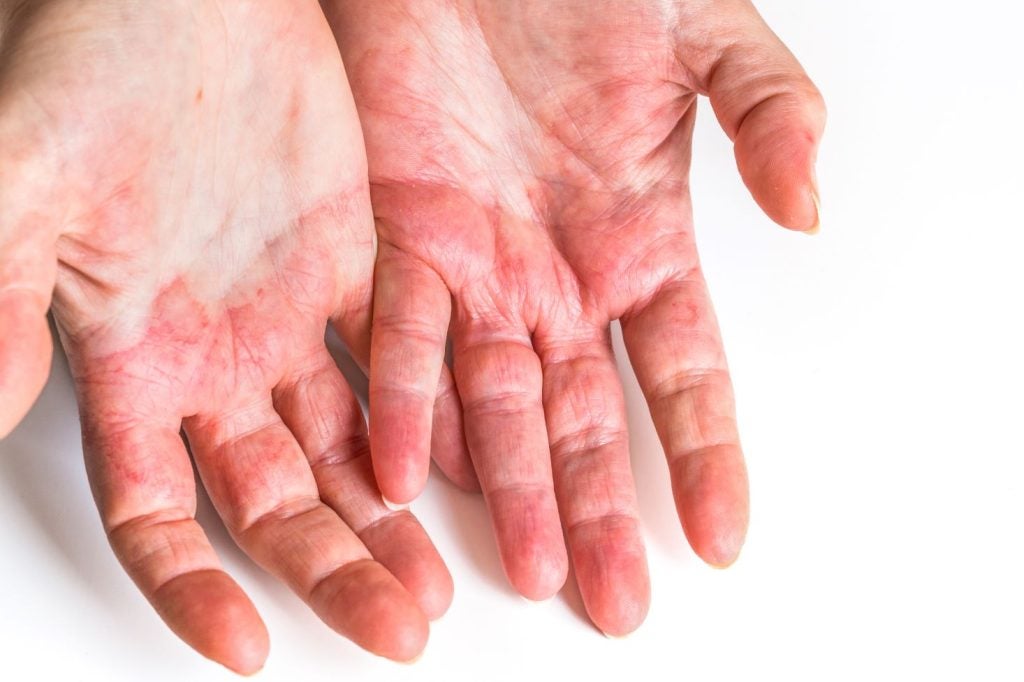
Unmet needs in the chronic hand eczema treatment landscape
Chronic exposure to irritants, allergens, and repetitive hand washing are established risk factors for chronic hand eczema.

Veeva introduces new platform for streamlined clinical trial execution
The offering consists of solutions Veeva Vault Clinical Suite, Veeva SiteVault Free and MyVeeva for Patients.
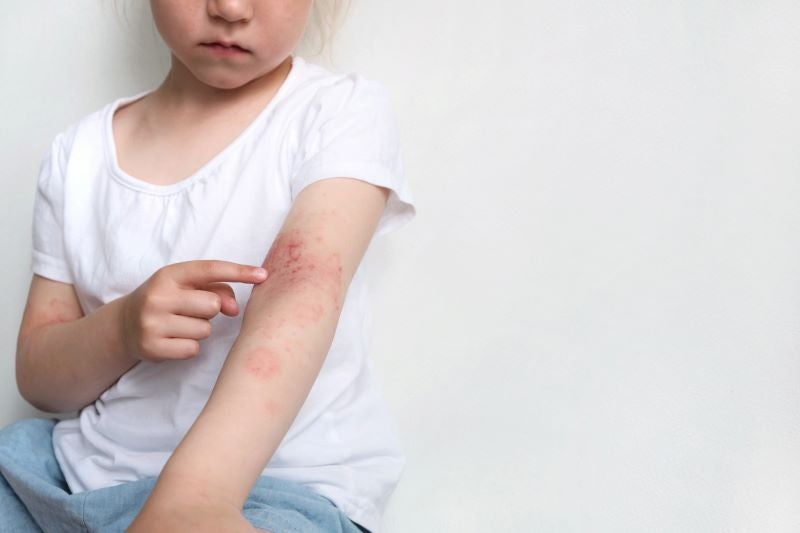
Spotlight on paediatric patients for atopic dermatitis
children with AD will soon receive more effective treatment options, as more developers begin expanding their investigations into this patient group.
Pipeline Moves: Trial completions in Duchenne muscular dystrophy and chronic urticaria
The Clinical Trials Arena team also investigates missed endpoints in wound infections, and terminations in head and neck cancer and liver cancer.
