Understand the impact of the Ukraine conflict from a cross-sector perspective with the Global Data Executive Briefing: Ukraine Conflict
Pfizer has announced that the company will not commence any new clinical trials in Russia amid the Ukraine crisis. The company will also halt participant recruitment in trials that are underway in Russia.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Working with the Food and Drug Administration (FDA) and other regulatory agencies, Pfizer will transition all trials underway to various other study centres outside of the country.
Furthermore, the company will offer the required drugs to the patients who are currently enrolled in trials, as part of its commitment to prioritise patients.
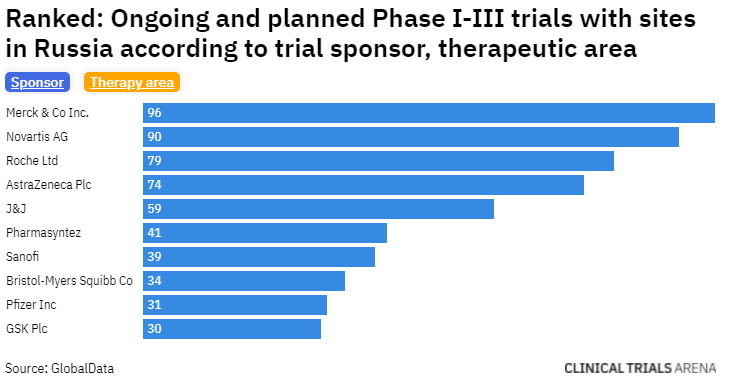
Previously, Clinical Trials Arena reported on 2 March that one of Russia’s top recruiting clinical trial sites paused recruitment of new patients in studies sponsored by overseas companies. We also did an analysis on how many clinical trials are at risk in Ukraine (24 February) and in Russia (7 March). In our Russia analysis, Pfizer ranks ninth in the top 10 pharma companies with an ongoing or planned Phase I-III trial in Russia.
Pfizer does not own or manage any manufacturing sites in Russia, thus all potential investments with suppliers in the country to expand manufacturing capacity will be halted by the company.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe company concluded that a voluntary halt in medicine flow to Russia would violate its foundational principle of putting patients first.
Talking about the humanitarian supply of therapies to Russia, Pfizer said in a press statement: “As with all previous instances, for humanitarian reasons, medicines were excluded from these sanctions.
“Ending delivery of medicines, including cancer or cardiovascular therapies, would cause significant patient suffering and potential loss of life, particularly among children and elderly people.”
Continuing the delivery of treatments to Russia however does not imply that the company will carry out business as normal in the country, Pfizer added.
In addition, all the profits of the company’s subsidiary in Russia will be donated to causes that offer humanitarian support to Ukrainians.
This donation is apart from Pfizer’s already announced commitments to Ukraine.
The latest development comes after the company’s CEO Albert Bourla told CBS News that it has no intention to make additional investments in Russia amid the Ukraine crisis.