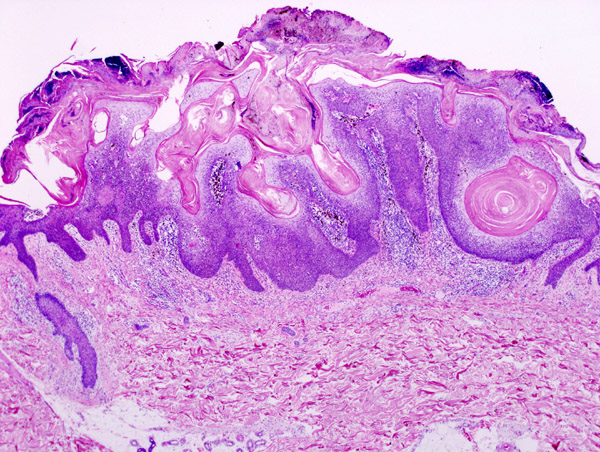
US-based specialty pharmaceutical firm Aclaris Therapeutics has started the first clinical study of its lead product A-101, a topically applied therapy, currently being investigated in adult patients for the removal of seborrheic keratosis, one of the most common types of benign skin tumours.
The study is designed to assess three concentrations of A-101 compared with placebo in a double-blinded, placebo-controlled, clinical trial of 36 patients with seborrheic keratosis.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The company expects to release the results from the first clinical study in the first half of 2014.
Aclaris chief operating officer Christopher Powala said the study will assess the tolerability and safety, as well as initial efficacy of A-101 in removing seborrheic keratosis lesions.
"There are currently no FDA-approved treatments for this condition and A101 has the potential to address this unmet medical need," Powala said.
Thomas Jefferson University clinical professor of dermatology Guy Webster said seborrheic keratoses are disfiguring and often uncomfortable.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData"An effective, FDA-approved therapy with the ability to minimise the risk of scarring or pigmentary changes would be a real step forward for patients and physicians," Webster saod.
Currently under development for topical treatment for both medical and aesthetic indications, A-101 is administered through topical route.
An estimated 83 million people in the US are affected by seborrheic keratosis and these benign (non-cancerous) skin tumours affect a majority of middle-aged to older individuals and impact men and women equally.
Seborrheic keratosis usually presents as slowly growing lesions that may vary in colour from flesh-coloured to yellow, tan, brown, or black and sometimes they resemble warts, moles, or skin cancers.
At present, seborrheic keratosis lesions are treated using invasive methods like cryosurgery, electrosurgery, curettage, or surgical removal which often result in pigmentary changes and/or scarring at the treatment site.
Image: Micrograph of a seborrheic keratosis. Photo: courtesy of KGH.





