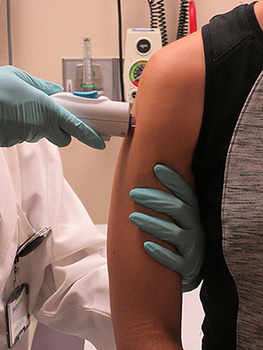
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), has launched a VRC 319 clinical trial of a vaccine to prevent Zika infection.
The NIAID Zika virus investigational DNA vaccine consists of a small, circular piece of DNA known as plasmid, designed to contain genes that code for proteins of the Zika virus.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
After the vaccine is injected into the arm muscle, the cells analyse the gene and make Zika virus proteins, which self-assemble into virus-like particles.
An immune response is issued on the particles, including neutralising antibodies and T-cells.
The DNA vaccine is free from any infectious material and hence does not pose any infection threat of the Zika virus of the vaccinated individual.
NIAID VRC director John Mascola said: “A team of scientists here at NIAID worked tirelessly to rapidly develop this vaccine for clinical testing.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“DNA or gene-based vaccines induce antibodies, but they also can activate the cell-mediated immune response, which ultimately could yield strong and durable protection against disease.”
The study is an attempt undertaken by the US government in the wake of Zika virus outbreak in the country.
The Phase I VRC 319 clinical trial is to include 88 healthy adult volunteers who will be divided into four groups of 20 people and injected with the vaccine fluid into their arm muscle.
After each vaccination, the clinicians will observe the participants for about 30 minutes to determine any adverse reactions.
Participants will be handed a diary card to keep a track of their temperature and any symptoms for the next seven days while being at home.
The participants will be followed up within a 44-week time period after the first vaccination for the clinicians to determine the safety of the vaccine following its administration.
During the follow-up visits, the review team will examine blood samples to determine immune response to the vaccine.
The patient data will be reviewed by the study team on a daily and weekly basis to monitor safety, and a Protocol Safety Review Team will also conduct formal interim safety reviews.
NIAD is additionally planning to commence a Phase II trial next year in countries affected by the Zika virus.
Image: Volunteer injected with NIAID Zika virus investigational DNA vaccine. Photo: courtesy of NIAID.





