Oxford BioMedica, a gene-based biopharmaceutical company, has concluded a Phase I/II study to evaluate the safety, efficacy and dose evaluation of ProSavin in patients with mid-stage Parkinson’s disease (PD) who are experiencing reduced benefit on L-DOPA equivalent therapy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
ProSavin employs the company’s LentiVector gene delivery technology to deliver the genes for three enzymes: aromatic amino acid decarboxylase, tyrosine hydroxylase and GTP-cyclohydrolase 1 that are required for the synthesis of dopamine.
The study investigated three ascending dose levels (1x, 2x and 5x) in a total of 15 PD patients at the Henri Mondor Hospital in Paris, France, and at Addenbrookes Hospital in Cambridge, UK.
In the Phase I/II trial of ProSavin, the primary endpoint is safety, and the secondary endpoint is efficacy as measured by the Unified Parkinson’s Disease Rating Scale (UPDRS) assessment at six months.
ProSavin has demonstrated a long-term safety profile, now up to 48 months post-treatment for the first two patients treated with a 1x dose, while all 15 patients treated have shown an improvement in motor function at the six-month efficacy endpoint relative to baseline.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAll six patients in the fourth and final cohort have reached their six-month assessment time point, reporting favourable safety profile with no serious adverse events and average motor function improvement of 30%, with a maximum of 41% in one patient.
The study’s independent Data Monitoring Committee also confirmed that the signals of improvements in motor function with decreased oral dopaminergic therapy observed to date are encouraging, particularly at the 5x dose.
Oxford BioMedica chief executive officer John Dawson said ProSavin is the first lentiviral vector-based treatment for Parkinson’s disease to be evaluated in a European clinical trial and the positive Phase I/II data supports further clinical development.
"We are currently evaluating a more potent formulation of ProSavin to ensure the greatest chance of success in randomised Phase II studies and increase the commercial opportunity for this novel product," Dawson added.
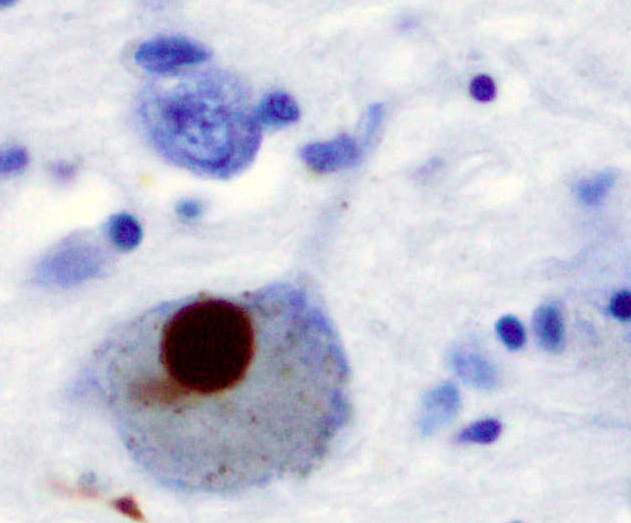
Image: A Lewy body (stained brown) in a brain cell of the substantia nigra in Parkinson’s disease. The brown colour is positive immunohistochemistry staining for alpha-synuclein. Photo: Marvin 101.





