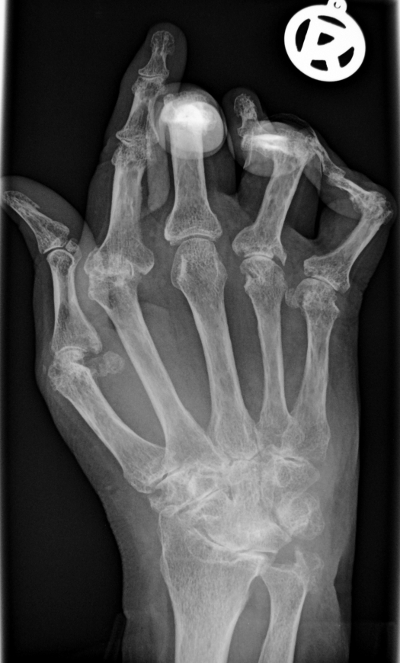
Clinical-stage biopharmaceutical company Protalex has reported positive interim review of phase 1b trial of PRTX-100 in rheumatoid arthritis (RA) by an independent data safety monitoring committee.
The multicentre Phase 1b randomised study of PRTX-100 in combination with methotrexate or leflunomide is continuing enrolment and increasing the dose for RA subjects.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
In the dose-escalation study, the PRTX-100 dose for subjects in the current treatment group is 12.0 micrograms/kg, which is eight times higher than that of the initial starting dosage of 1.5 micrograms/kg.
The primary objective is to evaluate the safety and tolerability profile of intravenous PRTX-100 administered weekly over five weeks in subjects with active RA on methotrexate or leflunomide therapy.
Determining the effects of PRTX-100 on measures of disease activity, assessing the immunogenicity and evaluating the pharmacokinetic (PK) parameters after repeated doses, and identifying possible relationships between the immunogenicity of PRTX-100 and safety, PK and efficacy parameters are the secondary objectives.
Around 40 subjects are expected to be enroled in the sequential dose-escalation phase into five cohorts ranging from 1.5 micrograms/kg to 18.0 micrograms/kg of PRTX-100, or placebo, per protocol.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataUp to 12 additional subjects might follow the dose-escalation phase for cohort expansion at a selected dose.
The study will increase the dose until occurrence of either dose limiting side effects or completion of the final cohort.
Enrolment is presently being carried out at five study sites across the US.
Image: X-ray of the hand in rheumatoid arthritis. Photo: courtesy of Bernd Brägelmann.





