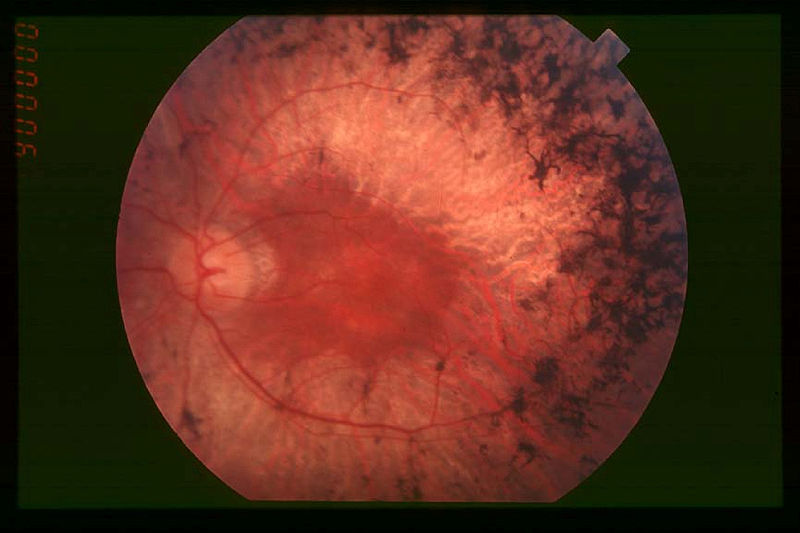
QLT has announced the positive preliminary results from its international multi-centre Phase 1b proof-of-concept clinical trial of QLT091001, used for the treatment of Retinitis pigmentosa (RP).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
In the open-label multi-centre Phase 1b clinical study, 17 subjects aged 6 to 55 years with either RPE65 or LRAT mutations were randomised to receive a 40 mg/m²/day dose of QLT091001 once daily for seven days with post-treatment follow-up at seven, 14, and 30 days.
The study showed clinically meaningful changes in visual fields (VF) from baseline values, as well as improvements in visual acuity (VA), which are evaluated using Goldmann Visual Fields (GVF) and best-corrected visual acuity respectively.
Following a single seven-day course of treatment with QLT091001, the average retinal areas from baseline demonstrated considerable improvements of 34% at day seven, 29% at day 14 and 23% at day 30 in the evaluable subjects meeting GVF test criteria.
In the intent-to-treat analysis, the average retinal area from baseline improved by 22% at day seven, 16% at day 14 and 18% at day 30.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe trial also investigated small subsets of RP subjects for secondary effects, such as decreased retinal sensitivity, and reported notable and promising improvements in average sensitivity levels.
QLT president and chief executive officer Bob Butchofsky said the preliminary data is promising, and showed clinically meaningful benefits of QLT091001 in the treatment of debilitating inherited retinal diseases due to the mutations.
“As these results apply only to a single course of therapy, additional studies are underway to assess the impact of repeat courses of treatment with QLT091001 in these patients, as part of our ongoing evaluation of safety and duration of effect,” Butchofsky added.
“Receipt of the final results of this broad ranging study expected in the second quarter this year will allow us to work with the regulators in the US and the EU to make further drug development decisions on this program.”
Dr.Hendrik Scholl of the Johns Hopkins University Wilmer Eye Institute said: “Following single-course treatment with QLT091001, the Retinitis pigmentosa patients in the clinical trial experienced a rapid and significant improvement in certain visual function parameters.”
QLT is a biotechnology company focused on the development and commercialisation of innovative ocular products that address the unmet medical needs of patients with inherited retinal diseases.
Image: Fundus of patient with mid-stage Retinitis pigmentosa. Photo: Christian Hamel.





