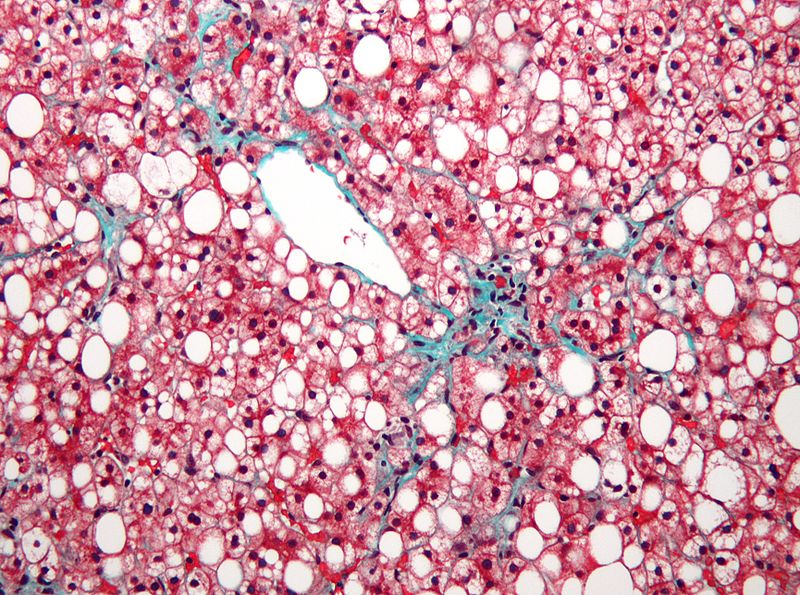
Raptor Pharmaceutical has dosed the first patient in its Phase 2b trial evaluating the safety and potential efficacy of RP103 for the treatment of non-alcoholic steatohepatitis (NASH), an advanced form of non-alcoholic fatty liver disease (NAFLD) in children.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The first patient was dosed with RP103, Raptor’s proprietary delayed-release microbead formulation of cysteamine bitartrate, at the Clinical and Translational Research Institute at the University of California, in San Diego, US.
Principal investigator for the NASH Clinical Research Network Pediatric Clinical Center, and Rady Children’s Hospital Fatty Liver Clinic director, Jeffrey Schwimmer, said NAFLD is the most common serious complication of childhood obesity.
"We are seeing a concerning increase in the number of adolescents with advanced liver disease, creating an urgent need to find an effective treatment for NAFLD," Schwimmer added.
"The results of a small pilot study were sufficiently encouraging and we are excited about the opportunity to test cysteamine in the form of RP103 as a treatment for NAFLD in children in this large, national study."

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataRaptor is conducting the Cysteamine Bitartrate Delayed-Release for the Treatment of Non-alcoholic Fatty Liver Disease in Children (CyNCh) trial within a cooperative R&D agreement with the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health.
The double-blind, placebo-controlled trial will enrol a total of 160 paediatric participants at ten US centres in the NIDDK-sponsored NASH Clinical Research Network.
The study will evaluate whether 52 weeks of treatment with RP103 in children reverses damage caused by NASH as measured by changes in NAFLD Activity Score, which is the primary objective of the CyNCh trial.
Secondary endpoints include blood markers for liver health, including alanine transaminase and aspartate transaminase, in addition to safety and tolerability.
Raptor president Ted Daley said: "The first patient dosed is an important milestone and we are pleased that the CyNCh clinical trial is underway."
Image: Micrograph of non-alcoholic fatty liver disease (NAFLD). Photo courtesy of: Nephron.





