 StemCells has started Phase I/II trial of its HuCNS-SC product candidate in dry age-related macular degeneration (AMD) at the Retina Foundation of the Southwest’s (RFSW) Anderson Vision Research Center in Dallas, Texas, US.
StemCells has started Phase I/II trial of its HuCNS-SC product candidate in dry age-related macular degeneration (AMD) at the Retina Foundation of the Southwest’s (RFSW) Anderson Vision Research Center in Dallas, Texas, US.
The dose-escalation study is designed to evaluate the safety and preliminary efficacy of HuCNS-SC cells, a purified form of human neural stem cells, as a treatment for dry AMD, referred to as geographic atrophy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
David Birch, RFSW chief scientific and executive officer, the Rose-Silverthorne Retinal Degenerations Laboratory director and study’s principal investigator, said that dry AMD was the most common form of macular degeneration and has a highly debilitating impact on the quality of life.
"Transplanting neural stem cells to protect photoreceptors in patients diagnosed with AMD is an innovative but logical approach, well supported by the company’s recently published preclinical data," he said.
In the open-label trial, which is expected to enrol 16 patients, HuCNS-SC cells will be administered by means of a single injection into the space beneath the retina in the most affected eye.
Conventional and advanced methods of ophthalmological assessment will be used to analyse patients’ vision at predetermined intervals over a one-year period to assess safety and signs of visual benefit.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe patients will then be followed for an additional four years in a separate observational study, according to the company.
The company’s preclinical research has shown that HuCNS-SC cells can directly be transplanted in the central nervous system with no sign of tumour formation or adverse effects.
HuCNS-SC cells were shown to protect host photoreceptors and preserve vision in an animal model of retinal disease in a rat by the Royal College of Surgeons (RCS).
StemCells CNS Program vice president and head Stephen Huhn said: "Our published preclinical data provides a strong rationale for this approach in dry AMD and we hope to replicate these results in this clinical trial."
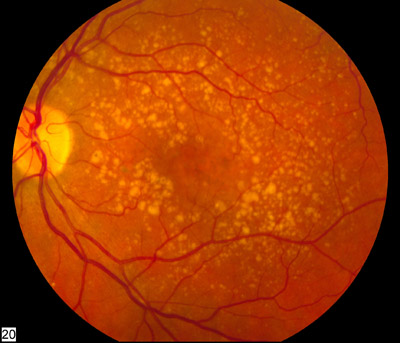
Image: A fundus photo showing intermediate age-related macular degeneration. Photo: National Eye Institute of the NIH.





