Threshold Pharmaceuticals has startedd Phase II trial to assess the clinical efficacy and safety of investigational hypoxia-targeted drug, TH-302, to treat patients with melanoma.
A range of biomarkers will also be assessed in the study such as serum, tumour biopsy, and PET imaging hypoxia biomarkers useful in predicting treatment outcomes and associated with tumour response to TH-302 therapy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
University of Toronto assistant professor Anthony Joshua said hypoxia is believed to be an important therapeutic target for patients with metastatic melanoma.
"Targeting hypoxic melanoma cells may help in slowing tumour progression and treatment resistance and has the potential to be an adjunct to current therapies," Joshua said.
Merck, Threshold’s partner for the development of TH-302, will fund 70% of development costs of the single-arm, multi-centre study.
Around 40 patients with advanced melanoma will be administered with 480mg/m² TH-302 per week on a 28-day cycle.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe primary endpoint is three-month progression-free survival, while secondary endpoints are response rate, duration of response, overall survival, safety and evaluation of potential imaging, serum, and tissue biomarkers associated with tumour response and predict for efficacy and safety of TH-302 therapy.
Threshold chief medical officer Dr Tillman Pearce said: "This Phase 2 study holds strategic importance for the overall TH-302 development program in potentially broadening therapeutic applications for TH-302 as well as facilitating potentially important biomarker research."
An increased potential for invasiveness, metastasis, and treatment resistance is exhibited by the cells in the hypoxic regions. Hypoxia may also suppress the immune response to cancer.
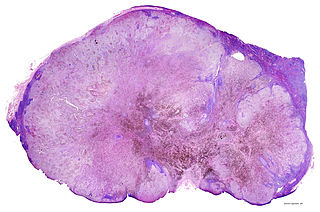
Image: Lymph node with almost complete replacement by metastatic melanoma. Photo: courtesy of Gabriel Caponetti, MD.





