The wave of biosimilars in oncology began with the EMA’s approval of the first antineoplastic biosimilar, Medice Arzneimittel Pütter’s Abseamed (epoetin alfa), in 2007. Oncology biosimilars currently account for the largest proportion of biosimilar therapeutics in the market. To date, the EMA has recommended the approval of 88 biosimilars, with 34 of those within oncology. Although the US was slower to join the biosimilar market, it quickly gained speed. The FDA has
currently approved 39 biosimilars, with 22 falling under oncology.
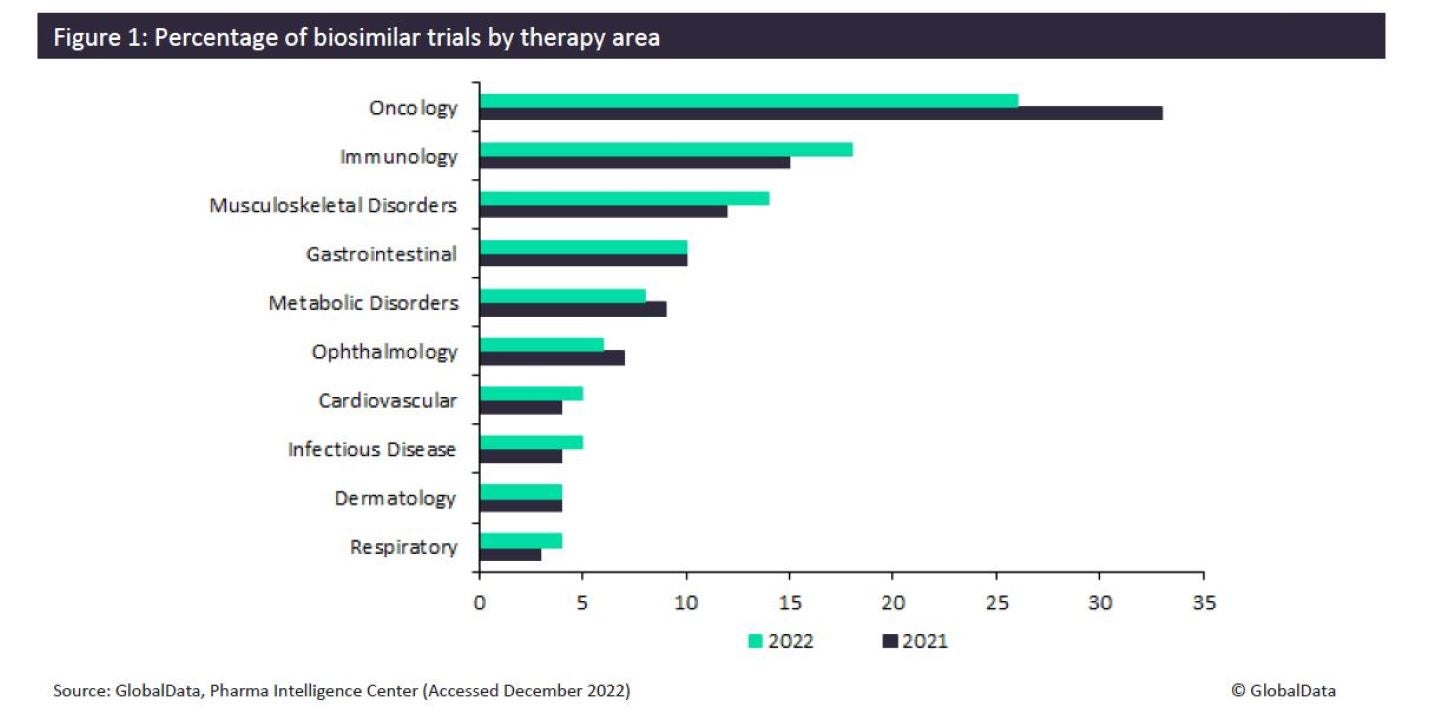
Biosimilar drugs are biological medicines that are extremely similar to another biological medicine that has already been licensed for use. The significantly lower prices of biosimilars make them an appealing alternative to branded biologics. Because of this, biosimilars increase patients’ access to advanced treatments, leading to improvements in clinical outcomes. Although biosimilars are highly represented in oncology, their presence is felt less in other therapy areas. However, data collated from GlobalData’s Trial Intelligence platform indicates a decrease in the proportion of biosimilar trials initiated for oncology, and an increase in trials among seven of the top ten biosimilar therapy areas from 2021 to 2022 (Figure 1).
Biosimilar drugs are crucial to supporting the UK National Health Service’s (NHS’s) affordability, which allows it to remain accessible to everyone. Similarly, since the FDA’s first biosimilar drug approval in 2015, biosimilars have generated more than $13 million in savings for patients and the US healthcare system. As biosimilars in other therapy areas gather more momentum, they will contribute to meeting the demand for additional treatment options. Additionally, GlobalData forecasts that the expansion of biosimilars outside of oncology indications will continue to drive growth in the biopharmaceutical industry due to an increased need for outsourced manufacturing. According to McKinsey’s Biosimilar market model, this growth is set to reach $60 billion by the end of the decade.





