Chimeric antigen receptor-T-cell (CAR-T) therapy is a form of immunotherapy that involves genetically modifying a patient’s T-cells to recognise and attack cancer cells.
T-cells are a type of immune cell that can detect and destroy abnormal cells in the body.
T-cells are collected from a patient’s blood, modified in a lab to produce a CAR that targets a specific protein on the surface of cancer cells, and then the cells are reinfused back into the patient’s bloodstream.
Once in the body, the CAR-T cells seek out and attack cancer cells that express the targeted protein, leading to tumour cell death.
CAR-T therapy has shown promising results in treating certain types of blood cancers such as leukaemia and lymphoma and is being studied in other types of cancer as well.
Most US Food and Drug Administration (FDA)-approved CAR-T cell therapies target the antigen CD19.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe first CAR-T therapy to be approved by the FDA was Novatis’ Kymriah, which was approved in August 2017 for the treatment of children and young adults with relapsed or refractory B-cell acute lymphoblastic leukaemia.
Kymriah targets the CD19 protein on the surface of cancer cells.
Since then, other CAR-T therapies have been approved for different types of cancer, including YESCARTA for certain types of non-Hodgkin lymphoma and TECARTUS for
mantle cell lymphoma.
Both YESCARTA and TECARTUS were developed by Kite Pharma, a subsidiary of Gilead Sciences.
The efficacy of CAR-T therapies can vary depending on the type of cancer being treated and other factors such as patient characteristics and disease stage.
However, CAR-T therapies have shown promising results in clinical trials for certain types of blood cancers.
It is important to note that CAR-T therapies can also have significant side effects and careful patient monitoring is necessary to manage these risks.
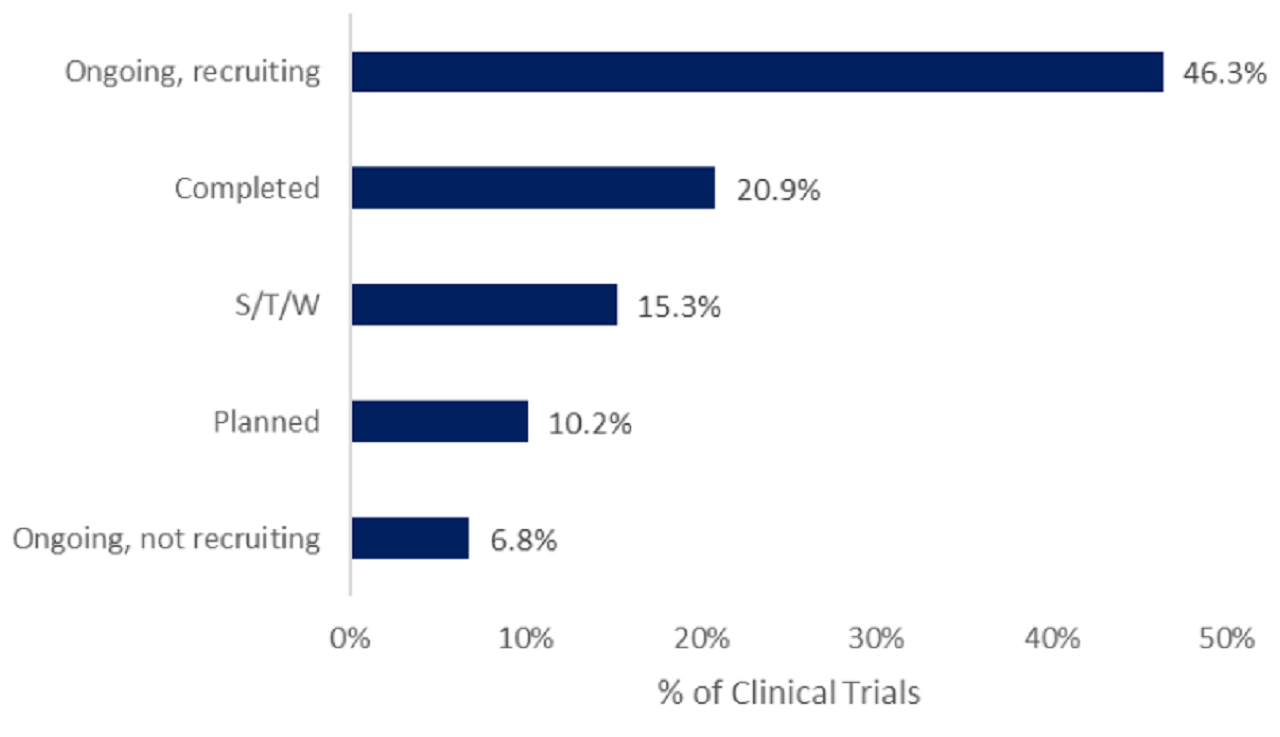
According to GlobalData’s Clinical Trials Database, CAR-T therapies peaked in 2022. reaching 271 trials for the year.