According to the World Health Organization, Coronaviridae or ‘coronaviruses’ are a large family of viruses that can cause illnesses ranging from the common cold to respiratory symptoms, fever, cough, shortness of breath, and breathing difficulties. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and even death. Two human coronaviruses, Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS), have been known to frequently cause severe symptoms. Now, a new strain that has not been previously identified in humans, the 2019 novel coronavirus (2019-nCoV), is emerging.
2019-nCoV is a Betacoronavirus, like MERS and SARS, all of which have their origins in bats. It was first detected in Wuhan City, Hubei Province, China. Chinese health officials have reported thousands of cases in China, and have noted the virus can spread from person to person. Cases are now being reported in a growing number of international locations, including the US. The first confirmed instance of person-to-person spread with this virus in the US occurred on January 30, according to the Centers for Disease Control and Prevention.
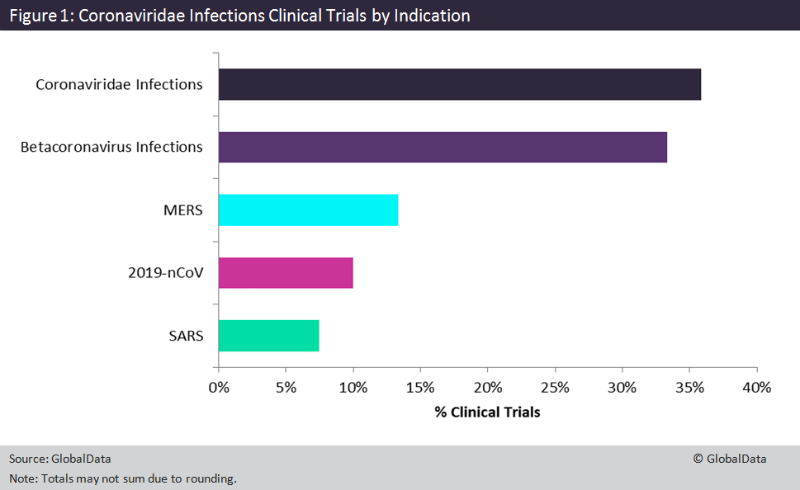
GlobalData looked at clinical trials captured in the Clinical Trials Database as of 4 February 2020 for Coronaviridae infections and specific infections such as MERS and SARS. The largest percent of trials, 69.2%, are investigating treatments for general Coronaviridae or Betacoronavirus infections, but the other 30.8% are investigating specific therapies for MERS, SARS, or 2019-nCoV, as shown in Figure 1. A total of 13.3% of trials are indicated for MERS, with 50% in Phase I development, 43.8% currently ongoing, and 18.8% planned. There have been no cases of SARS reported worldwide since 2004, so the 7.5% of trials with this indication are either completed or withdrawn.
Currently, 10% of these clinical trials are in response to the recent 2019-nCoV outbreak. Of these, 66.7% are planned to be initiated and the other 33.3% are already ongoing recruiting. At 41.7%, the majority are in Phase IV, followed by 33.3% in Phase 0 and 25% in Phase III. All sponsors of these 2019-nCoV trials are institutions located in China, besides one, which is a company-sponsored trial by Gilead Sciences. The Gilead trial is also taking place in China, specifically in Wuhan, and is a Phase III trial for patients with mild to moderate pneumonia caused by 2019-nCoV. The intervention is remdesivir (GS-5734), an experimental treatment for the Ebola virus infection, SARS, MERS, and nipah and zika virus infections. The drug candidate is a small molecule prodrug of adenine nucleotide analogue and acts by blocking the viral RNA replication process. The number of clinical trials indicated for 2019-nCoV is expected to rise as this outbreak continues.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData