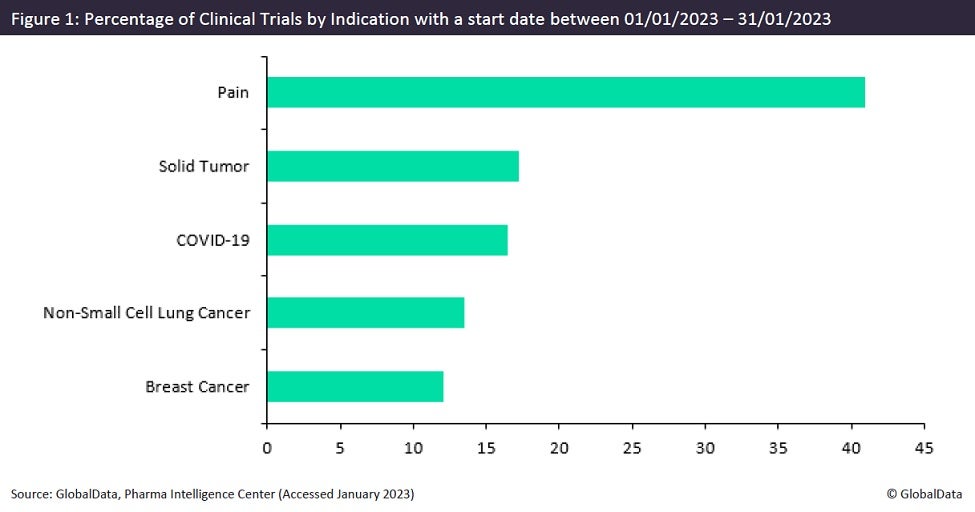
Figures obtained from GlobalData’s Trials Intelligence platform show that pain is the top indication being investigated in clinical trials initiating this January (Figure 1). A 2012 study by Nahin published in The Journal of Pain investigating the prevalence and severity of pain in the US discovered that 55.7% of American adults reported having pain in the last three months, with 11.2% living with chronic pain. So, it is no surprise that the opioid hydrocodone is one of the most prescribed medicines in the world. However, the opioid epidemic plaguing the US and Canada has produced a combined death toll of almost 600,000 over the last two decades with no signs of slowing. Many pharmaceutical companies have realized that a radical transformation in pain treatment is long overdue.
Tris Pharma is one of the pharmaceutical companies pioneering an alternative to traditional opioids with the novel drug cebranopadol. In December 2022, the company’s Phase IIb clinical study derived positive results showing cebranopadol was significantly less addictive, yet comparably efficacious to opioids. More recently, Wex Pharmaceuticals enrolled its first patient in the Phase IIb clinical trial evaluating the efficacy and safety of its novel drug Halneuron in the treatment of chemotherapy-induced neuropathic pain. As Halneuron does not cross the blood brain barrier, it is exempt from common side effects of opioids, most notably addiction, yet is more potent than standard analgesic agents including aspirin and morphine. Both Tris Pharma and Wex Pharmaceuticals are making vital steps towards addressing patients’ unmet needs for non-opioid pain therapies.
Pain is one of the greatest challenges faced in clinical trials. Although the demand for novel pain solutions couldn’t be more pressing, drugs targeting pain have some of the lowest trial success rates. GlobalData’s Drugs Intelligence platform reports that of the pain drugs that successfully made it to market, 16% were withdrawn. Furthermore, most marketed pain drugs are reformulations of existing compounds. The lack of objective pain measurements, safety issues, and varying tolerances are some of the main factors hindering pain clinical trials and subsequently the development of novel analgesic drugs. So, it is equal cause for celebration and trepidation awaiting further updates from Tris Pharma and Wex Pharmaceuticals on their formidable journeys.